
Bronze transformed human civilization as we moved from the Stone Age to the Bronze Age. Its outstanding durability and workability changed everything. The bronze melting point ranges between 1,562°F (850°C) and 1,922°F (1,050°C), based on the alloy’s makeup.
Standard bronze alloys melt at about 1,675°F (913°C). The temperature changes substantially depending on the metal’s composition. Bronze combines 80-90% copper with 5-25% tin. This mixture melts at lower temperatures than pure copper at 1,984°F (1,084°C). High-strength nickel-bronze alloys can reach temperatures up to 2,012°F (1,100°C). Metalworkers must understand bronze’s melting point because impurities and manufacturing methods can alter its melting behavior. These changes affect the metal’s structural strength and workability.
Understanding Bronze as a Metal Alloy
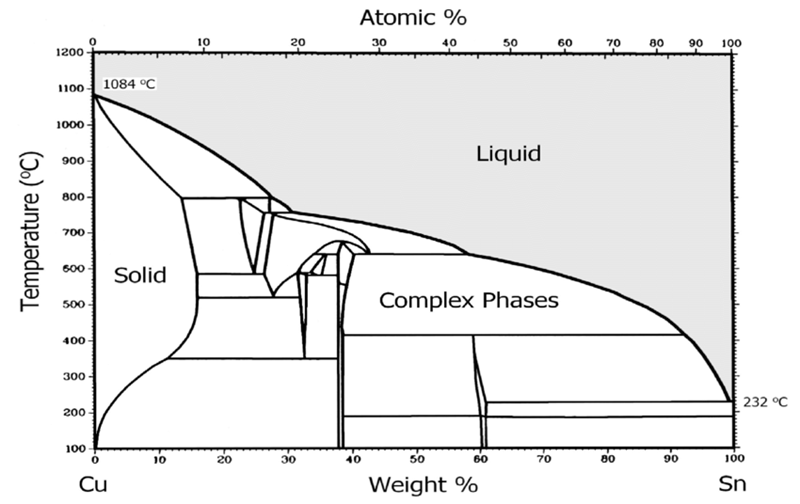
Bronze is a metal alloy where we combined copper with tin as the main alloying element. A traditional bronze mix contains about 88% copper and 12% tin. Historical artifacts show these proportions varied widely, ranging from 67% to 95% copper. The ratio between these two metals determines the alloy’s properties and how it melts.
Copper and Tin Composition in Bronze
Modern bronze still uses the copper-tin foundation but with exact formulations. The term “bronze” used to mean only copper-tin alloys, but now it overlaps with brass (copper-zinc alloys). Museums and historical texts now use “copper alloy” to avoid any confusion.
Standard bronze categories include:
- Tin Bronze: Contains up to 11% tin and 89% copper. This mix gives excellent strength, hardness, and resists corrosion well. These alloys work great in bearings, gears, and bushings.
- Commercial Bronze: Has about 90% copper and 10% zinc. This shows how modern bronzes sometimes use zinc instead of tin.
- Statuary Bronze: Contains roughly 97% copper, 2% tin, and 1% zinc. Artists and decorators use this type the most.
The amount of tin in bronze affects its melting point directly. More tin means a lower melting temperature but makes the bronze harder and stronger.
Role of Aluminum, Phosphorus, and Zinc in Bronze Alloys
Different elements added to the simple copper-tin mix create specialized bronze alloys with better properties.
Aluminum Bronze mixes 6-12% aluminum with copper, and sometimes includes iron, manganese, nickel, and silicon. This mix resists corrosion amazingly well, especially in seawater. The strength of aluminum bronze can match or beat medium carbon steel. That’s why it works so well in ship propellers and structural parts.
Phosphor Bronze combines copper, tin (about 11%), and a vital amount of phosphorus (up to 0.35%). Adding phosphorus removes oxygen and boosts the alloy’s stiffness by a lot. It also improves wear resistance and tensile strength. This makes phosphor bronze perfect for springs, electrical connectors, and bearings.
Manganese Bronze contains copper mixed with zinc, aluminum, iron, and 2.5-5.0% manganese. These bronzes are incredibly strong and resist wear well. They’re great for mechanical parts that need to handle high stress.
Silicon, bismuth, and lead are among other elements that can go into bronze. Each one changes how the bronze behaves mechanically and affects its melting temperature.
Factors That Influence the Melting Point of Bronze
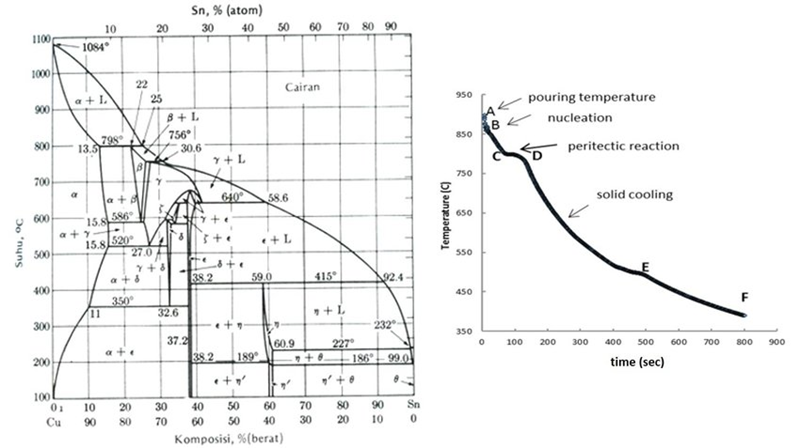
Metalworkers need to understand several factors that determine bronze’s melting point before they heat this versatile alloy.
Effect of Alloying Elements on Bronze Melting Temperature
The ratio of copper to tin mainly determines bronze’s melting temperature. When tin content is high (up to 20%), the melting point drops to around 850°C to 950°C (1562°F to 1742°F). This makes the metal flow better for casting complex components. Lower tin content (under 10%) pushes the melting point up to about 950°C to 1000°C (1742°F to 1832°F). This makes the metal harder and more durable.
Other alloying elements change these thermal properties. Aluminum bronze, with 6-12% aluminum, melts between 1027°C (1881°F) and 1038°C (1900°F). This gives it better strength at high temperatures. Silicon bronze has a melting point of about 1025°C. Adding lead drops the melting point to 800°C-900°C (1472°F-1652°F), which makes the metal easier to machine.
Impact of Impurities on Melting Range
Small amounts of impurities can change how bronze melts. Iron makes the melting point higher, while phosphorus lowers it and helps the metal flow better. The way bronze cools also matters. Fast cooling creates even structures with steady melting points. Slow cooling might separate components and change how the metal responds to heat.
Pressure and other environmental factors can cause small changes in melting temperatures during manufacturing. This means temperature control needs to be precise.
Solidus vs Liquidus in Bronze Phase Transition
Bronze doesn’t melt at one temperature like pure metals do. Instead, it changes phase between two points: the solidus and liquidus temperatures. The solidus is the highest temperature at which bronze stays completely solid. The liquidus is the lowest temperature at which it becomes fully liquid.
The “melting range” or “plastic range” falls between these temperatures. Here, bronze exists as a mix of solid and liquid phases. This temperature range is vital for metalworkers because it affects how metal flows and how strong joints become during casting or brazing. Alloys with narrow melting ranges flow better. These need tighter gaps between joined parts, usually 0.002″-0.006″.
Bronze Melting Point Range and Common Alloy Types
Bronze’s exact melting point changes based on its alloy composition. Metalworkers need to know these differences when they pick materials for casting or fabrication.
Typical Bronze Melting Point in Celsius and Fahrenheit
Standard bronze melts between 850°C to 1000°C (1562°F to 1832°F). These temperatures are lower than pure copper at 1085°C (1985°F), but higher than tin at 232°C (450°F). A traditional bronze mix with 88% copper and 12% tin usually melts at 950°C (1742°F).
Each bronze composition has its temperature sweet spot. Bearing bronze melts at 977°C, which makes it perfect for parts that need to handle friction well. Silicon bronze contains small amounts of silicon with copper and melts at about 1025°C (1868°F). This higher temperature makes it great for marine uses where you need strong corrosion resistance.
Melting Point of Tin Bronze vs Aluminum Bronze
Tin bronze and aluminum bronze show clear differences in how they react to heat:
Tin bronze melts between 870°C and 950°C (1600°F to 1742°F). More tin content (up to 20%) leads to a lower melting point, which helps create detailed castings easily.
Aluminum bronze, with its 6-12% aluminum content, needs much higher temperatures to melt, from 900°C to 1040°C (1652°F to 1904°F). Most aluminum bronzes melt between 1027°C (1881°F) and 1038°C (1900°F). These higher temperatures help make it stronger and better suited for hot environments.
High-Strength Nickel Bronze Melting Characteristics
Nickel-aluminum bronze alloys show unique melting patterns that make them ideal for tough jobs. These metals turn completely liquid at 1054°C (1930°F) and start to solidify at 1038°C (1900°F). Some types melt at slightly lower temperatures, becoming liquid at 1004°C (1840°F) and starting to solidify at 982°C (1800°F).
The gap between melting and solidifying temperatures creates a “plastic range” where the metal stays semi-solid. This feature helps control complex casting jobs better during cooling.
Manganese bronze melts between 865°C and 890°C (1589°F to 1634°F). It offers excellent mechanical strength at lower melting temperatures, which saves energy during casting.
Comparing Bronze with Brass and Copper
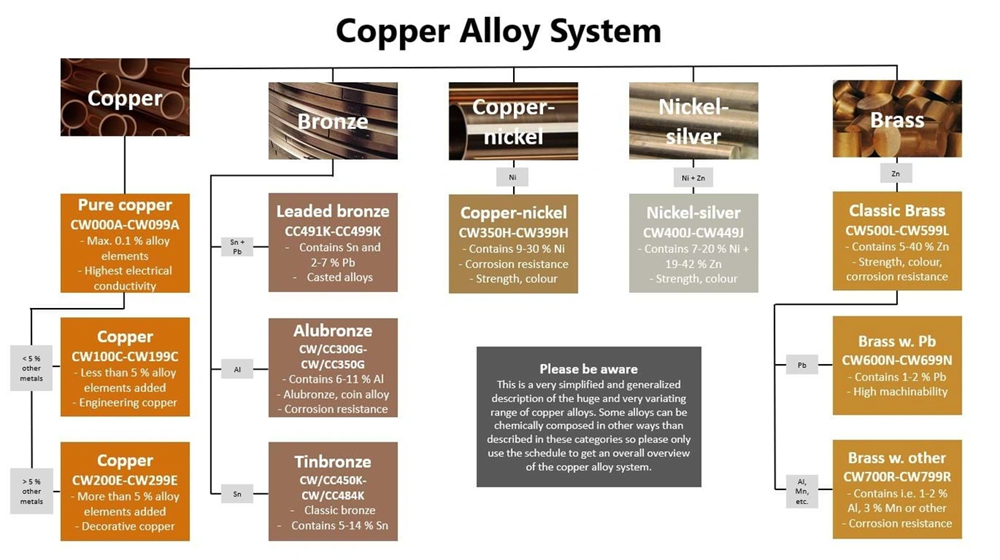
The melting characteristics of bronze, brass, and copper make them unique and affect how industries use and process them. Metalworkers need to know these thermal differences to pick the right alloy for their projects.
What Temperature Does Brass Melt Compared to Bronze?
Brass melts at lower temperatures than bronze, which creates different advantages during fabrication. Standard brass has a melting point between 900°C to 940°C (1652°F to 1724°F). Yellow brass turns liquid at 905°C to 932°C (1660°F to 1710°F). Red brass needs higher heat, melting between 990°C to 1025°C (1810°F to 1880°F).
Bronze typically melts at 913°C (1675°F), though this changes based on its makeup. Most bronze mixtures become liquid between 950°C to 1050°C. The zinc in brass causes this difference by lowering its melting point compared to bronze’s tin content.
Melting Point of Copper vs Bronze
Pure copper melts at much higher temperatures than bronze. It needs heat between 1083°C to 1085°C (1981°F to 1985°F). This is roughly 170°C more than what standard bronze alloys require. Bronze melts at lower temperatures because it mixes various elements with copper.
On top of that, special bronze types behave differently when heated. Aluminum bronze liquefies between 1027°C to 1038°C (1881°F to 1900°F), which comes close to copper’s range. Manganese bronze turns liquid at cooler temperatures, from 865°C to 890°C (1590°F to 1630°F).
Use Case Differences Based on Melting Behavior
These thermal differences shape how we use these metals. Brass’s lower melting point makes it easier to shape without special heating tools. This quality makes brass perfect for decorative items. Yet working with brass can be tricky since zinc releases toxic fumes during casting.
Bronze’s medium melting range helps create detailed castings. Its better heat resistance compared to brass makes it the top choice for high-temperature bushings.
Copper’s high melting point can make it harder to shape. However, this same quality gives copper excellent electrical conductivity, making it perfect for wiring. Copper stays more stable at high temperatures than its alloys, which helps when you need something to keep its shape in hot conditions.
Conclusion
Understanding Bronze Melting Points: Critical Knowledge for Metalworking Success
Bronze alloys show amazing versatility with melting points ranging from 850°C (1,562°F) to 1,050°C (1,922°F). This range gives metalworkers plenty of flexibility to select the right bronze formulation they need. The copper-to-tin ratio determines these thermal characteristics, and higher tin content usually brings down the melting temperature.
Of course, bronze’s thermal behavior is different from other copper alloys. Pure copper melts at a much higher 1,085°C (1,985°F), while bronze is easier to process and still keeps its excellent structural properties. Brass melts at lower temperatures than bronze because zinc affects its thermal behavior.
Metalworkers need to think over the key difference between solidus and liquidus temperatures. This “plastic range” where bronze stays in a semi-solid state is vital during casting operations. It affects flow characteristics and the quality of final components. Each bronze formulation has its own range width, and narrower ranges usually create better casting fluidity.
The simple copper-tin foundation changes a lot when you add other elements. Aluminum bronze has higher melting points, making it great for high-temperature uses. Adding lead lowers the melting point and makes the material easier to machine. Even tiny amounts of impurities can change bronze’s melting characteristics, which shows why quality control matters so much during manufacturing.
Bronze has stayed relevant through history because its melting point hits the sweet spot – low enough to cast practically yet high enough to stay strong in tough applications. This property, along with bronze’s exceptional durability and resistance to corrosion, explains why this ancient alloy still plays key roles in modern engineering and art.
Metalworkers who understand these thermal properties know how to pick the best bronze type, set the right processing parameters, and match them to final applications. Each bronze formulation’s specific melting point ends up being a vital factor that shapes production efficiency, component quality, and performance.
FAQs
Q1. What is the typical melting point range for bronze? The melting point of bronze generally falls between 850°C (1,562°F) and 1,050°C (1,922°F), depending on the specific alloy composition. Standard bronze alloys often melt around 913°C (1,675°F).
Q2. How does the composition of bronze affect its melting point? The copper-to-tin ratio in bronze significantly influences its melting point. Higher tin content typically lowers the melting temperature, while additional alloying elements like aluminum or lead can further alter the melting characteristics.
Q3. What are some unique properties of bronze related to its melting behavior? Bronze has a “plastic range” between its solidus and liquidus temperatures where it exists in a semi-solid state. This characteristic is crucial for casting operations as it affects flow properties and the quality of the final component.
Q4. How does bronze’s melting point compare to other copper alloys? Bronze generally melts at a lower temperature than pure copper (1,085°C or 1,985°F) but at a higher temperature than most brass alloys. This makes bronze easier to process than copper while maintaining excellent structural properties.
Q5. Why is understanding bronze’s melting point important for metalworkers? Knowledge of bronze’s melting point is crucial for metalworkers as it directly influences production efficiency, component quality, and the alloy’s suitability for specific applications. It helps in selecting the right bronze formulation and optimizing processing parameters.
