Stainless steel, one of the most commonly used metals in industries of all types, can actually rust. This fact surprises many people who assume this popular material never corrodes. The name “stainless steel” comes from its ability to resist rust in normal conditions, though it isn’t completely safe from corrosion. A material needs at least 10.5% chromium to qualify as stainless steel, which creates a protective barrier against oxidation.
The specific alloy determines stainless steel’s iron content. A thin, stable film protects the metal when its chemical elements interact with oxygen from water and air. This passive layer, sometimes just a few atomic layers thick, acts as a shield. It stops further corrosion by blocking oxygen and water from reaching the metal surface. Stainless steel can still corrode in six different ways: general, galvanic, intergranular, pitting, crevice, and stress corrosion cracking. The iron in the alloy reacts with water and oxygen to form hydrated iron oxide, which leads to rust formation under certain conditions. The protective passive layer can break down due to several factors like abrasion, chemical exposure, heat exposure, oxygen depletion, and galvanic reactions. These factors determine what makes stainless steel corrode.
How Stainless Steel Resists Rust at the Molecular Level
Stainless steel’s amazing resistance to corrosion comes from tiny molecular interactions at microscopic levels. Regular steel oxidizes quickly when air and moisture touch it. Stainless steel works differently – it creates a special protective barrier that guards the metal underneath.
Chromium Oxide Passive Layer Formation
The secret to stainless steel’s corrosion resistance lies in an incredibly thin film that appears naturally on its surface. This protective coating, known as the passive layer, forms when the chromium in the alloy meets oxygen from its surroundings. The film measures just one to three nanometers thick – about a few atoms deep. This tiny layer works powerfully to block oxygen and moisture from reaching the iron below.
The chromium oxide film has an amazing self-healing property. Any scratch or damage to the surface triggers an immediate reaction between the exposed metal and oxygen to rebuild the protective layer [4]. This automatic healing process keeps protecting the metal without needing any outside help.
Role of Chromium Content in Corrosion Resistance
Steel needs at least 10.5-12% chromium to earn the “stainless” label. This minimum amount ensures enough chromium exists to create a complete protective oxide layer. The metal becomes more resistant to corrosion as its chromium content goes up beyond this baseline.
Other elements make this protection even better. Adding more than 11% chromium, at least 8% nickel, or including molybdenum improves resistance to corrosion by a lot. This lets manufacturers create many grades of stainless steel that work best in specific environments.
Why Stainless Steel is Not Completely Rust-Proof
Yes, stainless steel can corrode under certain conditions. The protective layer works well, but can’t handle some environmental challenges. Exposure to chlorides, acidic conditions, physical damage, and iron particle contamination can break down this protective film.
Austenitic stainless steel contains at least 16% chromium and fights rust better than ferritic and martensitic types. But even stainless steels with high chromium content might corrode if they stay too long in harsh conditions.
What Causes Stainless Steel to Rust in Real-World Conditions

Stainless steel faces many environmental challenges that can compromise its corrosion resistance in real-world applications. This “stainless” material can still rust under certain conditions, despite its protective chromium oxide layer.
Chloride Exposure and Pitting Corrosion
Chloride ions pose one of the biggest threats to stainless steel’s integrity. These ions break through the passive layer and create localized breakdown points that turn into pits. The risk of corrosion increases by a lot in environments with higher chloride concentrations, especially with higher temperatures. Studies show that failures occur in environments with all but one of these chlorides at 10 ppm. The material faces especially dangerous conditions in coastal areas, places using deicing salts, and industrial settings with chloride-bearing compounds. Pitting corrosion can spread faster once it starts and forms deep cavities that might completely penetrate the meta.
Galvanic Corrosion from Dissimilar Metals
Galvanic corrosion happens when stainless steel touches a less noble metal, and water acts as an electrolyte. This electrochemical process needs three specific conditions: two metals with different corrosion potentials, electrical contact between them, and an electrolyte that connects both surfaces [10]. The surface area ratio between metals plays a crucial role in corrosion severity. The anode corrodes faster in moist environments when stainless steel (cathode) has a much larger surface area than the connected less noble metal (anode) [11]. So, carbon steel fasteners deteriorate faster in stainless steel structures exposed to moisture.
Mechanical Abrasion and Passive Layer Damage
Stainless steel becomes vulnerable to corrosion when its protective layer suffers physical damage. The material stays vulnerable until repassivation occurs after the passive film gets scratched or abraded. Iron particles from tools previously used on carbon steel can speed up this process. On top of that, abrasive cleaning methods might damage the passive layer without removing contaminants, just spreading them across the surface.
Heat-Induced Scaling and Weld Decay
“Heat-affected zones” (HAZ) form during welding and make stainless steel prone to intergranular corrosion, known as “weld decay”. This happens at temperatures between 425°C and 870°C when chromium combines with carbon to form chromium carbide at grain boundaries. Areas next to grain boundaries lose chromium and become vulnerable to selective attack. The sensitization process creates a localized galvanic cell where grain boundaries act as anodes while grain interiors become cathodic.
Contamination from Iron Particles
Free iron contamination causes stainless steel corrosion more often than you might think. Rust forms when carbon steel particles touch stainless steel surfaces and deposit free iron that oxidizes. Mixed metal tools, grinding nearby carbon steel, and industrial environments with atmospheric iron commonly cause this issue. Tests using potentiodynamic polarization show that iron contamination reduces the localized corrosion resistance of stainless steels by a lot. Chemical or electrochemical treatments work best to remove free iron from stainless surfaces, as mechanical cleaning methods usually fall short.
Types of Stainless Steel Corrosion You Should Know
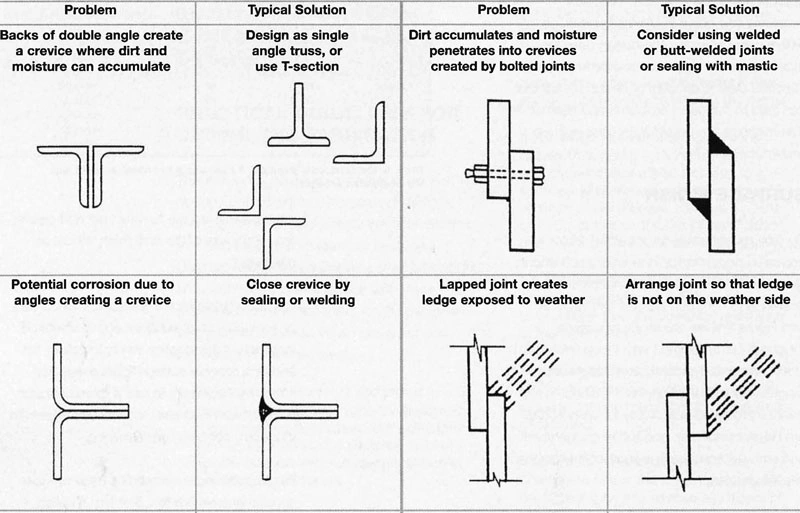
Knowing specific corrosion mechanisms helps us spot weaknesses in stainless steel applications. The most resistant grades still face unique corrosion challenges under different conditions.
General Corrosion in Low pH Environments
Stainless steel behaves differently from ordinary carbon steels and rarely corrodes evenly across its surface. The protective passive layer breaks down completely in highly acidic environments with pH values below 1.0. Specific chemicals, mostly acids, attack the entire surface at once. Hydrochloric and sulfuric acids pose the biggest threats at certain concentrations and dissolve the metal’s surface layer steadily. Metal loss spreads evenly in this type of attack. This creates a predictable deterioration pattern that can quickly affect structural integrity.
Crevice Corrosion in Low-Oxygen Areas
Stainless steel loses its protective passive film in tight spaces where oxygen can’t circulate well. These oxygen-starved spots typically show up at fastener interfaces, under gaskets, or in poorly designed joints. The passive layer breaks down as oxygen levels drop inside these crevices. Chlorides build up and pH levels fall, which creates an aggressive microenvironment. The bare metal becomes exposed to faster attacks. Design choices are vital since using a higher-grade alloy alone might not eliminate this risk.
Stress Corrosion Cracking Under Tension
This rare but dangerous failure happens when tensile stresses combine with specific corrosive environments that often contain chlorides. The right mix of temperature, stress, and environment creates tiny cracks that spread faster. Sometimes, mechanical properties get destroyed within days instead of years. Swimming pools and hot water tanks often see this phenomenon. The tensile forces don’t need to be extreme. The residual stresses from manufacturing or welding are enough to start the cracking.
Intergranular Corrosion from Improper Heat Treatment
Chromium combines with carbon to form chromium carbide precipitates along grain boundaries when stainless steel heats between 450-850°C. This “sensitization” process strips nearby areas of corrosion-resistant chromium and creates microscopic galvanic cells. The chromium-depleted zones corrode first after exposure to corrosive media. Eventually, entire grains can break loose from the surface. This “weld decay” often happens during welding operations. Using low-carbon “L” grades or stabilized alloys with titanium or niobium prevents this issue.
Best Practices to Prevent Rust on Stainless Steel

Stainless steel needs strategic approaches in design, fabrication, maintenance, and material selection to stay rust-free. These best practices help maintain the passive layer that keeps corrosion away.
Design Considerations to Minimize Crevices
Tight joints and areas without proper oxygen circulation can lead to crevice corrosion. Designs should eliminate tight joints where possible. Welding works better than mechanical joints for water applications. Non-immersion applications benefit from flexible inert washers or construction sealants. Tanks need elevation from the floors. Full drainage capabilities and smooth, rounded corners inside vessels make cleaning easier and reduce moisture-trapping sediment buildup.
Fabrication Guidelines to Avoid Cross-Contamination
Cross-contamination stands as the biggest problem in unsuccessful stainless steel fabrication. The best approach is setting up separate areas just for stainless steel work. Equipment used for both carbon and stainless steel needs a full cleaning between uses. Stainless steel or non-metallic tools should clean surfaces because carbon steel wire brushes leave iron particles that start corrosion. Keep welding, cutting, or grinding of carbon steel away from stainless steel components.
Routine Cleaning with Non-Abrasive Tools
Clean regularly to remove contaminants that harm the passive layer. Mild detergents and soft cloths work best – stay away from chloride-containing or abrasive cleaners. Stainless steel needs thorough drying after cleaning. Standing moisture blocks the protective chromium oxide film from regenerating. Stubborn stains need specialized stainless steel cleaners with oxalic acid. These remove calcium deposits without surface damage.
Use of Passivation and Pickling Treatments
Pickling uses nitric and hydrofluoric acid solutions to remove heat-affected zones and chromium-depleted layers. Passivation follows pickling with milder acids that remove surface contaminants and help the passive film form. Both treatments get rid of free iron contamination that regular mechanical cleaning misses.
Choosing the Right Grade for the Environment
Your operating environment substantially affects stainless steel’s performance. Grade 316 with molybdenum gives better resistance to pitting corrosion in chloride-rich or marine environments. Austenitic stainless steels (300 series) are the foundation of overall corrosion resistance and formability. Applications that need welding do better with alloys 304L or 347. These prevent cracking and corrosion at weld sites.
Conclusion
Understanding Stainless Steel’s True Nature
Stainless steel deserves its reputation as a corrosion-resistant material, though it’s not the rust-proof metal many believe. A protective chromium oxide layer serves as the main defense against oxidation and creates a molecular shield just atoms thick. This remarkable barrier heals itself, which explains why stainless steel performs better than many other metals under normal conditions.
The material’s integrity faces several challenges. Chloride exposure, galvanic reactions, mechanical damage, heat treatment problems, and iron contamination pose the most important threats to the passive layer. Knowledge of these vulnerabilities helps prevent early failures in critical applications.
Environmental conditions determine how different corrosion mechanisms affect stainless steel. Acidic environments trigger general corrosion, while oxygen-starved spaces develop crevice corrosion. The combination of tensile forces and corrosive substances causes stress corrosion cracking. Manufacturing or welding’s improper heat treatment typically results in intergranular corrosion.
Stainless steel’s corrosion prevention needs a multi-faceted approach. Good design eliminates potential crevices and moisture traps. Careful fabrication stops carbon steel particle contamination. Quick removal of harmful deposits protects the passive layer. Chemical treatments like passivation and pickling restore the steel’s protective properties. The right grade selection for specific environments ensures peak performance.
This scientific understanding of stainless steel’s behavior guides better material selection, improved designs, and more effective maintenance protocols. Engineers and maintenance professionals can make smart decisions that extend service life and prevent failures from getting pricey. Stainless steel remains an excellent material choice when specified and maintained according to its true capabilities rather than misconceptions about its limitations.
FAQs
Q1. Can stainless steel rust? Yes, stainless steel can rust under certain conditions. While it’s more resistant to corrosion than regular steel, exposure to harsh chemicals, saltwater, or prolonged moisture can cause stainless steel to corrode.
Q2. What causes stainless steel to rust? Stainless steel can rust due to factors like chloride exposure, galvanic corrosion from contact with dissimilar metals, mechanical damage to its protective layer, heat-induced scaling, and contamination from iron particles.
Q3. How long does it take for stainless steel to rust? The time it takes for stainless steel to rust varies greatly depending on environmental factors, the grade of stainless steel, and maintenance practices. In ideal conditions, some types can last for hundreds of years, while in harsh environments, corrosion can occur much faster.
Q4. How can I prevent my stainless steel from rusting? To prevent rust, regularly clean stainless steel with mild soap and water, dry thoroughly after cleaning, avoid abrasive materials, use appropriate stainless steel cleaners, ensure proper ventilation, and consider applying protective coatings in harsh environments.
Q5. Is it possible to remove rust from stainless steel? Yes, rust can often be removed from stainless steel. For light surface rust, a paste made from baking soda and water or a cleaner containing oxalic acid can be effective. For more stubborn rust, mechanical or chemical removal methods may be necessary.
