
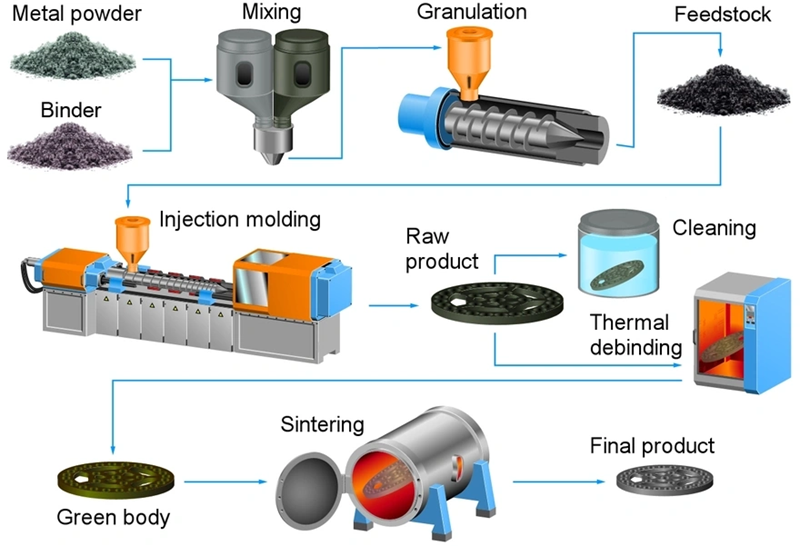
Debinding is the most time-consuming and critical stage in the metal injection molding (MIM) process, directly affecting the quality of the finished parts. MIM feedstock contains 40% or more binder by volume, unlike conventional powder metallurgy, which uses only a few percent of binder material. This requires a carefully controlled extraction process. Parts emerge in a “green” state about 20% larger than their final dimensions after the first molding.
Removing these binder materials creates major technical challenges. Manufacturers must balance complete binder extraction without damaging the molded components. The cost of binder removal runs roughly ten times higher than the cost of the binder itself, which shows how delicate this balance is. The three main approaches to debinding—thermal, solvent, and supercritical fluids—each work better for different binder compositions and desired part properties. Solvent extraction can be used to perforate the binder structure before thermal processing. In this piece, we’ll look at the key differences between solvent and thermal debinding methods to help manufacturers choose the best approach for their specific MIM applications.
Binder Composition and Its Role in Debinding
Binder systems are the foundations of metal injection molding (MIM) processes that help create complex metal parts with exceptional precision. These carefully mixed formulations keep metal particles together during molding and maintain structural integrity throughout later processing stages.
Polymer and Wax Components in MIM Feedstock
MIM feedstock builds on fine metal powder mixed with a multi-component binder system that will give a proper flow during injection. Metal powders make up 60-65% of the feedstock volume, while binders constitute the remaining 35-40%. This exact ratio will give a good flow during molding and maintain enough metal content to produce dense, strong final products.
Most binder systems combine primary and secondary components. Primary binders usually contain polypropylene (PP), polyethylene (PE), and polystyrene (PS) to provide structural stability and shape retention. Paraffin wax reduces viscosity and helps fill molds better during injection. Secondary binders such as stearic acid, polyethylene glycol (PEG), carnauba wax, and microcrystalline wax aid the debinding process and work as lubricants during molding.
Melting and Decomposition Temperatures of Common Binders
Successful debinding depends on understanding each binder component’s thermal properties. A typical MIM binder system shows different melting points for each material:
- Polymethyl methacrylate (PMMA): 157°C
- Polypropylene (PP): 165°C
- Paraffin wax: 60°C
- Stearic acid: 70°C
These components have different decomposition temperature ranges. Simple binder components break down between 154-557°C, though metal powders can change this range. Metal powder Ti-6Al-4V moves this range to 113-506°C, which suggests it might speed up the decomposition process.
Impact of Binder Selection on Debinding Strategy
Binder system choice shapes the best debinding approach. Paraffin wax melts at much lower temperatures than polymer materials, so many manufacturers use a sequential debinding strategy. They remove wax components first, then handle polymers later. This step-by-step method reduces the risks of deformation and defects during processing.
Polyoxymethylene-based systems like Catamold work well with catalytic debinding using nitric acid vapor at around 110°C. Polymer materials with higher melting points need thermal debinding in vacuum furnaces to stop metal powder oxidation.
Manufacturers must keep some binder after primary debinding to support the “brown part” until sintering starts. Finding the right balance between removing binder and maintaining structural support is vital for successful MIM processing.
Solvent Debinding Process in MIM
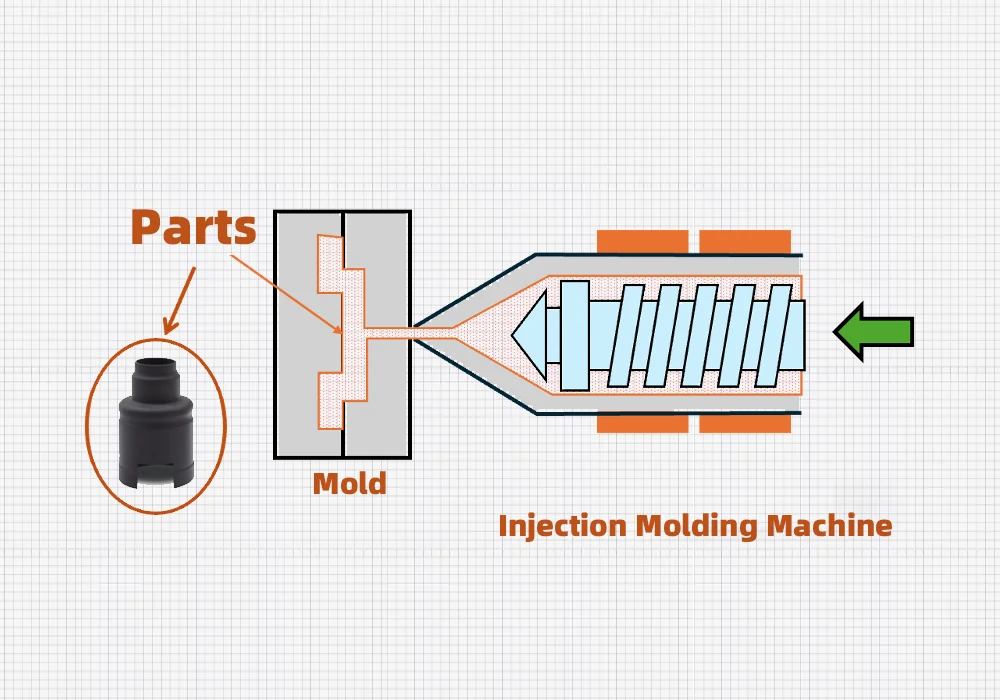
Image Source: IQS Directory
Solvent debinding is the quickest way to create interconnected pores in the MIM process while keeping parts intact. This technique removes specific binder components through dissolution and creates channels for later binder removal stages.
Solvent Types: Acetone, Heptane, and Water
MIM parts can be debound using several solvents, each with unique properties:
- Acetone and heptane are classic choices for hydrophobic binder systems that work well to dissolve paraffin wax components.
- Trichloroethylene gives good brown part strength but raises environmental concerns.
- Water has become more popular as an eco-friendly option, but it needs water-soluble or water-reactive polymers in the binder mix.
Many manufacturers now choose water-soluble binder compositions. These are easier to handle compared to organic solvents that need distillation and recycling after contamination.
Immersion vs Vapor Phase Solvent Debinding
Solvent debinding happens in two main ways:
Liquid immersion means putting green parts directly into heated solvent at 60°C, usually with constant stirring. This removes binders faster but can change solvent concentration over time and change debinding speeds.
Vapor phase debinding uses vaporized solvent in a controlled setting. The vapor goes through the green parts and dissolves binders evenly without surface problems. While this used to be slower than immersion, new vapor degreasing techniques have cut processing times from hours to just minutes.
Temperature Control for Optimal Wax Extraction
Temperature plays a key role in how well debinding works. Research shows that 60°C is the sweet spot for removing paraffin wax. At this temperature, you can remove about 74% of the wax in 240 minutes without warping the part. This temperature is close to paraffin wax’s melting point, which helps remove it quickly without causing damage.
Parts can crack, swell, or fail at higher temperatures above 70°C, even though initial extraction might be faster.
Diffusion and Solubility Factors in Solvent Debinding
Several factors determine how well solvent debinding works:
- Part shape and aspect ratio change debinding speeds
- The right solvent-to-feed ratio matters (7:1 by weight works best)
- Higher temperatures speed up molecular diffusion, but this needs careful control
The porous network created after solvent debinding helps gasses escape during thermal debinding later. This prevents common issues like blistering or cracking.
Thermal Debinding Process in MIM

Thermal debinding plays a vital role in MIM processing. Heat carefully removes binder components through decomposition and evaporation. The process demands precise control of multiple parameters to produce defect-free brown parts.
Furnace Atmosphere and Temperature Ramp Rates
MIM applications require gas-tight hot-wall retort furnaces. These systems work in inert atmospheres or under hydrogen in a vacuum to stop oxidation. Temperature control needs multiple holding phases at specific points. The thermal cycles work best with low-temperature removal from room temperature to 200°C for solvent extraction. High-temperature removal happens between 200-600°C for volatiles with higher boiling points. Backbone binder removal occurs at ≥600°C. Careful regulation of heating rates prevents trapped gases and ensures complete removal.
Gas Flow Requirements for Wax Vapor Removal
Gas flow dynamics optimize the debinding process. Effective wax vapor removal needs gas flow rates up to 25 times the hot zone volume per hour. Modern furnaces use specialized gas inlet diffusors. These direct flows horizontally through component layers. Special gas outlet plates ensure even distribution throughout the retort chamber. Even gas circulation prevents central channeling, and this is a big deal as it means that the debinding process works better.
Deformation Risks During Polymer Decomposition
Part deformation risks emerge during thermal degradation processes. Research shows feedstocks with more than 35.3 vol.% of depolymerization-type polymers degrade faster than those with more than 8.5 vol.% of random-type polymers. Thermal degradation gases from polyoxymethylene (POM) and polymethyl methacrylate (PBMA) can create cracks and holes. This happens when heating rates exceed 30°C/hr in the 270-300°C range. So, slow decomposition reduces deformation risks. However, rates that are too slow hurt productivity and might trigger unwanted side reactions.
Vacuum Furnace Use for Oxidation Prevention
Cold-wall retort furnaces with vacuum conditions stop oxidation during combined debinding and sintering processes. Horizontal vacuum furnaces deliver better results for debinding applications that need tight tolerances. These systems come with complete safety features. They include PLC controls with safety monitoring, gas monitoring, and automatic shutdown mechanisms. Metal powders may form oxides without proper oxidation prevention. These oxides can block subsequent sintering and harm the final part’s properties.
Catalytic Debinding and Hybrid Approaches
Catalytic debinding technology stands as a game-changer in the MIM industry. It processes materials up to 40 times faster than conventional thermal or solvent methods. This breakthrough has transformed production efficiency, especially when you have high-volume manufacturing needs.
Polyoxymethylene-Based Catamold Systems
BASF created the Catamold system in the early 1990s that employs polyoxymethylene (POM) as its main binder component. This polyacetal binder system gives excellent moldability and keeps its shape throughout processing. POM’s unique molecular structure has continuous oxygen-carbon chains that react quickly to acid attack. The chemical makeup allows direct conversion from solid to gas without becoming liquid first, which reduces the risk of part deformation.
Nitric Acid Vapor as Catalyst at 110°C
The catalytic process uses highly concentrated nitric acid vapor (>98.5% purity) as its primary catalyst. Traditional thermal debinding needs temperatures above the binder’s melting point. The catalytic process works at just 110°C, which sits well below POM’s melting range of 150-170°C. The acid vapor sparks a chemical reaction that breaks down the POM binder into formaldehyde units (CH₂O) with a boiling point of -21°C. These gas molecules escape through the porous outer layer and leave the powder particle structure intact.
Binder Removal Rate vs Part Thickness
The catalytic debinding moves at a steady linear velocity between 1-2 mm per hour at 110°C and creates well-connected pores throughout the part. Notwithstanding that, thicker parts slow down the debinding rate due to capillary condensation and diffusion limitations. Therefore, the gas flow through the debinding oven must be optimized to maintain quick processing rates in thicker components.
Residual Binder Support for Brown Part Strength
After catalytic debinding, about 10% of acid-resistant binder components stay within the part. This leftover material gives crucial structural strength to the brown part during pre-sintering handling. The acid-resistant components burn off during the first stages of sintering, usually at temperatures above 600°C. Keeping this backbone polymer prevents part collapse when moving between debinding and sintering operations.
Recent state-of-the-art developments include alternative catalysts like oxalic acid powder. This offers environmental and safety benefits over nitric acid while delivering similar debinding results. More importantly, new hybrid approaches that combine local debinding and sintering technologies have emerged. These streamline processes for metal parts while avoiding toxic agents and expensive post-processing equipment.
Conclusion
Debinding remains the most critical and time-intensive phase in the metal injection molding process. This phase directly determines final part quality and production efficiency. In this piece, we got into how different approaches tackle the fundamental challenge of extracting 40% binder volume without compromising structural integrity. Of course, each method offers distinct advantages based on specific manufacturing requirements and part geometries.
Solvent debinding excels at creating interconnected porosity networks while preserving delicate structures, especially when you have complex parts where deformation risks run high. On top of that, water-based systems have emerged as environmentally friendly alternatives to traditional organic solvents like acetone and heptane. Thermal debinding provides detailed binder removal but requires precise temperature control and specialized furnace environments to prevent oxidation and structural defects.
The progress toward catalytic processes using polyoxymethylene-based systems has accelerated production speeds by up to 40 times compared to conventional methods. This remarkable advancement highlights the continuous state-of-the-art within MIM technology. Hybrid approaches combining multiple debinding techniques often yield optimal results for challenging applications.
Manufacturers must think over several factors to select a debinding strategy: part geometry, binder composition, production volume, and final material properties. The ideal approach often involves a staged process that begins with solvent or catalytic debinding followed by thermal treatment. JH MIM brings nearly 20 years of experience to the Metal injection molding and Powder metallurgy industry. Their factories span more than 18000 square meters, equipped with world-class machinery and 150 skilled workers who deliver precision-engineered products to global customers.
Successful MIM processing depends on understanding the complex relationship between binder behavior and debinding parameters. Debinding costs approximately ten times more than the binder materials themselves. Yet this stage without doubt represents a worthwhile investment to achieve high-quality, complex metal parts with exceptional dimensional accuracy and material properties. Manufacturers who become skilled at these debinding techniques so position themselves for success in this growing field of advanced manufacturing.
FAQs
Q1. What is the purpose of debinding in Metal Injection Molding (MIM)? Debinding is a critical step in MIM that removes the binder materials used to hold metal particles together during molding. It creates a porous structure in the part, preparing it for sintering while maintaining its shape and integrity.
Q2. How does solvent debinding work in MIM? Solvent debinding uses liquids like acetone, heptane, or water to dissolve specific binder components. This creates interconnected pores in the part, facilitating further binder removal and preparing it for thermal processing.
Q3. What are the key considerations in thermal debinding for MIM parts? Thermal debinding requires precise control of furnace atmosphere, temperature ramp rates, and gas flow. It’s typically performed in inert or reducing atmospheres to prevent oxidation, with carefully managed heating cycles to avoid defects.
Q4. How does catalytic debinding differ from other methods? Catalytic debinding, often using polyoxymethylene-based systems, employs acid vapor (typically nitric acid) at lower temperatures (around 110°C) to rapidly depolymerize the binder. This method can be up to 40 times faster than conventional techniques.
Q5. Why is residual binder important after the primary debinding stage? A small amount of residual binder (about 10%) is intentionally left after primary debinding to provide structural support for the “brown part” during handling. This remaining binder is removed during the initial stages of sintering, preventing part collapse between processes.
