Metal Injection Molding (MIM) combines the flexibility of plastic injection molding with the strength of powdered metals to produce small, intricate parts for the medical field. The medical and dental segment accounts for approximately 14% of the global MIM market, with North America leading in regional application share.
| Aspect | Data / Insight |
|---|---|
| Medical & Dental segment share | Approximately 14% of the total global MIM market |
| North America MIM market share | 28% of the global MIM market |
| Medical & Dental applications | Includes surgical instruments, implants, and orthodontic brackets requiring miniaturization and biocompatibility |
MIM Usage supports the creation of complex shapes, high-density parts, and biocompatible components that meet strict medical standards. Manufacturers use stainless steel, titanium, and cobalt-chrome alloys to achieve the precision and reliability required for surgical instruments and implants.
Key Takeaways
- Metal Injection Molding (MIM) creates small, complex, and precise medical parts like surgical instruments and implants using strong, biocompatible metals.
- MIM improves medical device quality by reducing parts, cutting costs, and enhancing durability, precision, and surface finish for safer, reliable tools.
- The MIM process involves mixing metal powders with binders, molding, removing binders, and sintering to produce dense, high-quality components.
- Materials like stainless steel, titanium, and cobalt-chrome alloys ensure MIM parts resist corrosion, support biocompatibility, and meet strict medical standards.
- MIM faces challenges like size limits and complex steps but offers cost-effective, scalable production with design flexibility and strong regulatory compliance.
MIM Usage in Medical Device Manufacturing
Common Medical Devices Produced with MIM
Manufacturers rely on Metal Injection Molding to produce a wide range of medical devices that demand intricate designs and high precision. MIM Usage proves especially valuable for components that require tight dimensional tolerances and complex 3D geometries. Many medical and dental devices benefit from this technology, including orthodontic brackets, endoscopic device parts, and implantable components. Stainless steel, titanium, and cobalt-chrome alloys serve as the primary materials, offering mechanical strength and biocompatibility. The process allows for miniaturization and the creation of parts that would be difficult or impossible to manufacture using traditional methods. Medical devices produced with MIM often include surgical staples, orthopedic screws, dental implants, and specialized connectors. These parts must meet strict standards for reliability and safety, making MIM an ideal choice for the healthcare industry.
Surgical Instruments and Components Made by MIM
Surgical instruments require exceptional durability, precision, and ergonomic design. MIM Usage enables manufacturers to consolidate multiple components into single, integrated parts, reducing assembly time and improving overall quality. The following table highlights key performance improvements observed in surgical instruments made with Metal Injection Molding:
| Performance Aspect | Improvement / Feature | Example / Metric |
|---|---|---|
| Component Consolidation | Reduction in part count | From 12 parts to 3 parts (Surgical Stapler) |
| Manufacturing Cost | Cost reduction | 40% reduction (Surgical Stapler) |
| Assembly Time | Time reduction | 60% reduction (Surgical Stapler) |
| Quality Defects | Defect reduction | 85% reduction (Surgical Stapler) |
| Stapling Consistency | Performance improvement | 25% improvement (Surgical Stapler) |
| Weight Reduction | Weight savings | 35% reduction (Surgical Stapler), 50% (Biopsy Forceps) |
| Mechanical Properties | Pullout strength increases | 850N vs. 650N (31% increase) (Orthopedic Screws) |
| Fatigue Resistance | Endurance cycles | >5 million cycles without failure (Orthopedic Screws) |
| Dimensional Accuracy | Thread accuracy | ±0.02 mm consistency (Orthopedic Screws) |
| Surface Finish | Optimization for reduced tissue adhesion and smooth operation | <1 μm Ra surface finish (Biopsy Forceps) |
| Complex Geometries | Internal channels, undercuts, thin walls | Channels as small as 0.3-0.4 mm diameter; 0.5 mm wall thickness |
| Biocompatibility & Corrosion Resistance | Material selection and surface treatments enhance reliability and safety | Titanium alloys (ISO 10993 certified), Cobalt-Chrome alloys with corrosion rate <0.1 mm/year |
Surgical forceps, clamps, scalpel handles, and retractors are frequently manufactured using MIM. These instruments benefit from improved ergonomics, enhanced sterilizability, and consistent mechanical properties. MIM also supports the production of orthopedic screws and biopsy forceps, which require precise thread geometries and excellent surface finishes. Manufacturers achieve superior fatigue resistance and dimensional accuracy, ensuring that instruments perform reliably during critical procedures.
Real-World Examples of MIM in Healthcare
Healthcare providers and medical device companies have adopted MIM Usage to address the growing demand for advanced medical technologies. Real-world applications demonstrate the versatility and effectiveness of MIM in producing high-precision, durable, and complex medical components. Notable examples include:
- Biocompatible implants with intricate, precise, and durable designs that improve integration with the human body.
- Surgical instruments such as forceps, clamps, scalpel handles, and retractors benefit from enhanced ergonomics and precision.
- Orthopedic components and dental implants, leveraging MIM’s ability to create complex geometries and excellent surface finishes.
- Customized prosthetics that are lightweight, durable, and aesthetically improved for better patient comfort.
Case studies highlight successful collaborations between manufacturers and technology providers. For instance, Westminster Tool partnered with Mantle Inc. and Foster Corp. to develop medical-grade forceps using MIM and metal 3D-printed mold cavities. This project showcased rapid prototyping and efficient production of precise surgical instruments. Another example involves a medical company that used MIM to manufacture a new type of surgical knife, significantly enhancing sharpness and durability. Medical professionals praised the improved performance and reliability of these instruments.
The following table presents additional examples of medical components produced using MIM and their benefits:
| Medical Component | Application/Benefit |
|---|---|
| Compression Frame | Holds and aligns the targeting drill guide during tarsometatarsal fusion |
| K-Mount for Surgical Camera | Award-winning component for digital surgical cameras |
| Planetary Gear System | Integrated pinion gear and posts for surgical devices |
| Pusher | Used in ratcheting assembly for consistent placement of high viscosity adhesives |
| Wedge Blank | The smallest and most effective component for the endoscopic staple gun |
| Distal Channel Retainer | Complex, multi-level 17-4 PH stainless steel part formed via MIM |
| Stapling Device Shuttle | Stainless steel shuttle for “smart” stapling device in open and minimally invasive surgery |
| Pump Latch | Used in IV pumps |
| Tungsten Electrode | Used in surgical ablation devices for tissue removal at high temperatures |
| Endoscopic Device Parts | Four stainless steel MIM components, including articulation lock bar |
| Stainless Steel Shaft Assembly | Used in a novel surgical instrument for passing sutures through difficult-to-reach tissue |
These examples illustrate how MIM Usage drives innovation and quality in medical device manufacturing. The technology enables the production of reliable, high-performance components that meet the stringent requirements of modern healthcare.
The MIM Process for Medical Applications
Key Steps in Metal Injection Molding
The Metal Injection Molding process for medical applications follows a series of precise steps to ensure high-quality results:
- Feedstock Preparation: Technicians blend fine metal powders with thermoplastic binders, forming a uniform feedstock. This mixture ensures proper flow during molding.
- Injection Molding: The feedstock is heated and injected into a mold, creating a “green part” that holds the desired shape but lacks final strength.
- Debinding: Specialists remove the binder using solvent extraction or thermal methods. This step produces a fragile “brown part” with a porous structure.
- Sintering: The brown part is heated in a controlled furnace near the metal’s melting point. Metal particles fuse, densify, and shrink uniformly, resulting in a strong, dense component.
- Secondary Operations (Optional): Some parts undergo additional treatments, such as heat treatment, machining, or surface finishing, to meet strict medical specifications.
Note: Medical MIM often includes extra steps like hot isostatic pressing (HIP) to achieve near-full density and superior mechanical properties, especially for critical implants.
Materials Used in Medical MIM
Medical device manufacturers select materials that combine strength, corrosion resistance, and biocompatibility. Common choices include:
- Stainless Steel (316L, 17-4PH): Offers excellent strength and corrosion resistance, making it ideal for surgical instruments and implants.
- Titanium Alloys: Provide a high strength-to-weight ratio and outstanding biocompatibility, suitable for orthopedic and dental implants.
- Magnesium Alloys: Valued for biodegradability and compatibility with the human body.
- Cobalt-Chrome Alloys: Used for their durability and resistance to wear.
- Shape Memory Alloys: Employed in specialized devices requiring flexibility and precise movement.
Manufacturers use powders with high purity and controlled particle size to ensure consistent results. All materials must meet international standards, such as ASTM and ISO, and pass rigorous biocompatibility tests, including ISO 10993 and FDA guidelines.
Suitability of MIM for Medical Manufacturing
MIM suits medical manufacturing due to several factors:
- Ability to produce small, intricate, and complex parts with tight tolerances.
- Wide material selection supports diverse medical applications, from surgical tools to implants.
- High density and minimal porosity reduce the risk of bacterial contamination.
- Biocompatibility and corrosion resistance ensure safety and longevity in the body.
- Cost-effective net-shape production minimizes material waste, especially for expensive alloys.
- Enhanced process control and repeatability meet strict regulatory and quality standards.
These advantages make MIM a preferred choice for manufacturing advanced medical and surgical components.
Advantages of MIM Usage in Medical and Surgical Products

Precision and Design Complexity
Metal Injection Molding delivers unmatched precision and supports highly complex designs in medical and surgical products. Manufacturers use this process to create intricate geometries and micro features that traditional metalworking cannot achieve. Devices such as forceps, scissors, endoscopic tools, and stents require tight tolerances and detailed shapes. MIM achieves tolerances as low as ±0.001 inches, ensuring each part meets exact specifications. This level of accuracy is essential for device reliability and patient safety.
| Aspect | Details |
|---|---|
| Applications | Surgical instruments, drug delivery systems, diagnostic tools, and cardiovascular devices |
| Precision & Tolerances | ±0.001 to ±0.003 inches (±0.025 to ±0.076 mm) |
| Design Freedom | Thin walls, internal channels, fine surface finishes |
| Mechanical Properties | High tensile strength, hardness, corrosion resistance |
MIM enables the integration of multiple functions into single components, reducing assembly steps and potential failure points. This design flexibility enhances device efficiency and reliability.
Cost-Effectiveness and Production Scalability
MIM Usage offers significant cost savings, especially for high-volume production. The process reduces material waste to about 5%, compared to 30-40% in machining. For a 100,000-piece order, MIM costs $250,000, while CNC machining costs $615,000. The cost per part decreases as production volume increases, making MIM ideal for mass manufacturing.
- MIM supports medium- to high-volume production, including millions of parts annually.
- The process integrates multiple manufacturing steps, reducing lead times and costs.
- Automation ensures consistent quality and expands production capacity.
- MIM is suitable for disposable and reusable surgical instruments, meeting growing hospital demand.
MIM allows rapid design changes and supports innovation in medical technology, making it a scalable solution from prototypes to millions of units per year.
Biocompatibility and Material Properties
Medical devices require materials that are safe for the human body and perform reliably under stress. MIM uses biocompatible materials such as stainless steel, titanium, and cobalt-chromium alloys. These materials offer corrosion resistance, durability, and ease of sterilization.
| Material | Biocompatibility Advantages | Key Properties |
|---|---|---|
| Stainless Steel | Corrosion resistance, easy sterilization | Strong, low ion release |
| Titanium | Promotes osseointegration, low immune response | Lightweight, high-strength |
| Cobalt-Chromium | Suitable for long-term implants, high wear resistance | Hardness, low friction, corrosion resistant |
Surface properties, such as smooth finishes, reduce bacterial adhesion and enhance tissue compatibility. The MIM process produces parts with excellent surface quality, minimizing the risk of adverse reactions. These material properties ensure that implants and instruments remain safe and effective throughout their use.
Technical and Regulatory Considerations for MIM Usage
Quality Control and Consistency
Manufacturers implement strict quality control measures in Metal Injection Molding for medical devices. They select and test materials to guarantee reliability and patient safety. Advanced measurement technologies help manage design complexities and tight tolerances. Continuous process improvements support consistent results.
- Material quality and consistency remain critical throughout production.
- Sterilization and biocompatibility challenges are addressed by validating processes and choosing compatible materials.
- Compliance with FDA regulations and ISO standards, such as ISO 13485 for quality management and ISO 14971 for risk management, is mandatory.
Metal Injection Molding ensures consistency through a multi-step, tightly controlled process.
- Technicians formulate a homogeneous feedstock from fine metal powders and binders.
- The compounding process is tightly controlled for reliability.
- Precision injection molding shapes the parts.
- Debinding removes binders from molded parts.
- Sintering heats parts near their melting point, bonding metal particles and causing shrinkage.
- Sintering fixtures maintain dimensional accuracy.
- Collaboration with MIM experts during design enhances consistency.
- Scientific principles and experimental design rules create a stable, scalable process.
- Rigorous quality assurance and regulatory compliance ensure biocompatibility and performance standards.
Regulatory Compliance in Medical Manufacturing
Regulatory compliance plays a vital role in medical device manufacturing using MIM. Biocompatibility is essential, especially for implantable devices. Manufacturers must select materials that meet FDA and ISO requirements. Surface treatments optimize corrosion resistance and biological compatibility. Rigorous quality assurance systems maintain consistency and regulatory compliance across batches. Customization for patient-specific needs is also important. High levels of quality control are mandatory to ensure patient safety and device efficacy.
Design for Manufacturability and Collaboration
Design for manufacturability principles guide successful MIM Usage in medical devices. Engineers design parts under 30 grams with uniform wall thickness to optimize processing. They use radius corners and avoid sharp edges to prevent voids and tooling difficulties. Specialized tooling enables undercuts and threads, reducing secondary machining. Collaboration with MIM providers early in the design process ensures manufacturability and compliance.
- Sintering shrinkage of 15-20% is accounted for in mold design.
- Uniform wall thickness prevents warping and cracks.
- Fillets and radii reduce stress concentrations and improve material flow.
- Gates and parting lines are positioned to promote balanced flow and minimize defects.
- Supports or gussets prevent sagging during sintering.
Early and continuous partnership between design and manufacturing teams improves outcomes. This teamwork enables the production of complex, precise parts with excellent mechanical performance and surface quality. Integrating design for manufacturability with secondary processes enhances product reliability and reduces defects.
Challenges and Limitations of MIM Usage
Material and Process Constraints
Manufacturers face several material and process constraints when using Metal Injection Molding for medical devices. Specialized machinery increases equipment and tooling costs. The process involves multiple steps, including mixing, injection molding, debinding, sintering, and finishing, which extend lead times. Achieving consistent material properties such as uniform density and porosity remains challenging. Thin structures and sharp edges often prove difficult to mold, limiting design flexibility. Environmental concerns arise from hazardous binders and additives, requiring careful handling and disposal. Size and weight limitations favor small, intricate components over larger parts. Shrinkage during sintering, typically around 15% to 20%, demands precise mold scaling and process control to maintain dimensional accuracy.
Note: Metal Injection Molding produces a ‘green part’ that must undergo debinding and sintering. These steps can introduce variability in mechanical performance, especially for complex geometries.
Comparison with Other Manufacturing Methods
Metal Injection Molding offers unique advantages but also faces competition from other manufacturing methods. CNC machining provides superior control over fine features and surface finishes, making it suitable for parts with extremely tight tolerances. Investment casting supports larger components and complex shapes but may require more post-processing. Additive manufacturing enables rapid prototyping and customization, though it often lacks the material properties needed for long-term medical use. The table below compares key aspects:
| Method | Strengths | Limitations |
|---|---|---|
| Metal Injection Molding | Intricate shapes, high volume | Size limits, shrinkage control |
| CNC Machining | Precision, surface finish | Higher material waste, cost |
| Investment Casting | Large parts, complex forms | Longer post-processing |
| Additive Manufacturing | Rapid prototyping, customization | Material property constraints |
Addressing Common Obstacles
Manufacturers employ several strategies to overcome obstacles in Metal Injection Molding for medical devices:
- Advanced tooling solutions such as slides, lifters, and collapsible cores help mold complex geometries and undercuts.
- Secondary operations like machining and laser cutting add precision features that are difficult to achieve directly in molding.
- Insert molding and overmolding consolidate components and enable multi-material parts, reducing assembly steps.
- Early collaboration with tooling suppliers and emphasis on Design for Manufacturability balances complexity, cost, and manufacturability.
- Prototyping and additive manufacturing validate complex designs before full-scale production.
A medical device producer improved material stability by switching to a higher-grade resin and revising pre-treatment processes, which reduced defects and enhanced product integrity. Companies also expand engineering capabilities and integrate specialized processes, such as laser welding and silicon overmolding, to manage complex supply chains and ensure compliance with standards like ISO 13485 and FDA requirements.
Metal Injection Molding continues to transform medical device manufacturing by delivering precision, reliability, and innovation. Selecting the right process ensures devices meet strict healthcare standards and patient needs. The future looks promising, with the global MIM market in healthcare projected to more than double by 2034.
| Year | Market Size (USD) |
|---|---|
| 2024 | 1.88 billion |
| 2025 | 2.06 billion |
| 2034 | 4.58 billion |
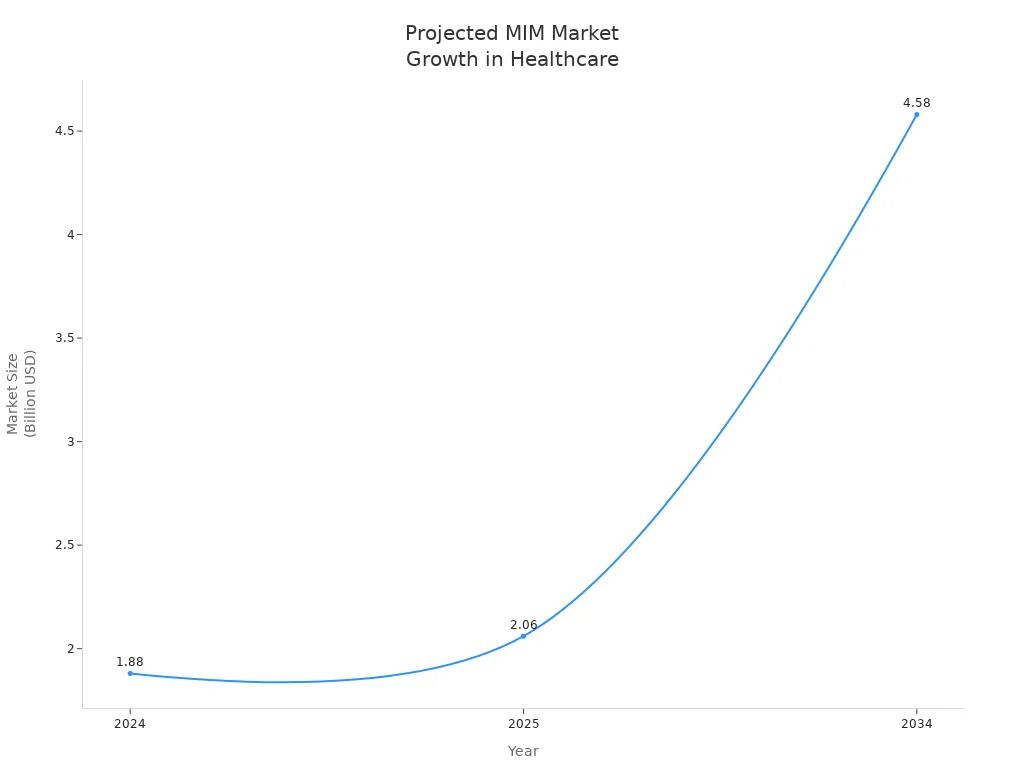
Industry leaders expect robust growth, driven by technological advances and rising demand for complex medical solutions.
FAQ
What types of medical devices benefit most from Metal Injection Molding?
Manufacturers use MIM for surgical instruments, dental implants, orthopedic screws, and endoscopic device parts. These products require complex shapes, high precision, and biocompatible materials. MIM enables efficient production of small, intricate components for advanced medical applications.
How does MIM ensure biocompatibility in medical components?
Engineers select certified materials such as stainless steel, titanium, and cobalt-chrome alloys. These metals meet international standards for biocompatibility. Rigorous testing and surface treatments further enhance safety for use inside the human body.
Can MIM produce custom or patient-specific medical parts?
MIM supports customization for patient-specific needs. Designers adjust molds and material choices to create unique geometries. This flexibility allows manufacturers to deliver tailored solutions for implants and specialized surgical tools.
What quality control measures do manufacturers use in medical MIM?
Manufacturers implement strict inspection protocols. They use advanced measurement tools to verify dimensions and surface finishes. Quality management systems, such as ISO 13485, ensure consistent production and compliance with regulatory standards.
Is MIM cost-effective for high-volume medical device production?
MIM offers significant cost savings for large production runs. The process reduces material waste and streamlines manufacturing steps. Automation and scalability make MIM ideal for producing millions of medical components annually.
