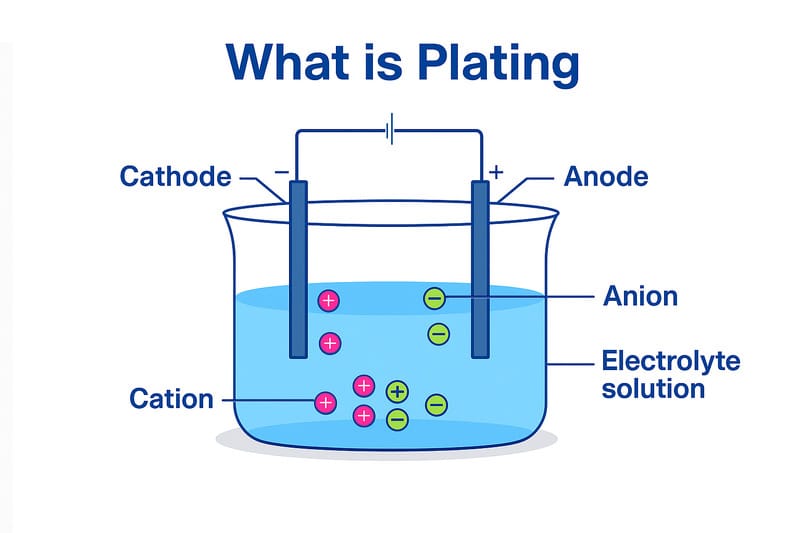
Plating, or what is plating?, involves applying a thin layer of material, often metal, to an object’s surface to enhance its functionality or appearance. This technique improves durability, resists corrosion, and elevates visual appeal. For instance, studies show that factors like the thickness of nickel coatings, ranging from 3% to 26.5%, directly influence uniformity and performance. Additionally, adhesion strength plays a critical role in maintaining durability in humid environments, while hydrophobic properties prevent corrosion. These qualities make plating indispensable across industries, from electronics to automotive manufacturing.
Key Takeaways
- Plating enhances the strength of materials, prevents rust, and improves their appearance. It is important in industries like cars and electronics.
- There are different ways to plate, like electroplating or electroless plating. Each method has special benefits for different uses.
- Cleaning and finishing steps during plating help the coating stick well. These steps also make the coating last longer and work better.
- Picking the right plating material helps stop rust and protects items. This can make products last much longer.
- Plating is useful and looks nice, so it’s used in factories and for things like jewelry people wear.
Why is plating important?
Plating plays a vital role in enhancing the functionality and appearance of materials, making it indispensable across various industries. Its importance lies in its ability to improve material properties, elevate aesthetic appeal, and extend the lifespan of products.
Enhancing material properties
Plating significantly improves the physical and chemical properties of materials. For instance, it enhances corrosion resistance, wear resistance, and electrical conductivity. These benefits are particularly valuable in industries such as automotive and electronics, where durability and performance are critical.
| Benefit | Description |
|---|---|
| Corrosion Resistance | Protects materials from rust and degradation, extending their lifespan. |
| Enhanced Electrical Conductivity | Aids in conducting electricity, crucial for electronic components. |
| Wear Resistance | Increases surface hardness, reducing damage from friction and abrasion. |
| Chemical Resistance | Shields materials from harsh chemicals and environmental conditions. |
The global plating market reflects this importance. In 2023, it was valued at $707.82 million and is projected to grow to $1,213.08 million by 2030, with a compound annual growth rate (CAGR) of 8%. Automotive applications dominate this growth, highlighting the demand for plating in enhancing material properties.
Improving aesthetic appeal
Plating transforms the appearance of objects, adding a polished and attractive finish. This is especially evident in jewelry, decorative items, and even food presentation. A consumer study revealed that aesthetic attributes, such as color, significantly influence preferences. For example:
- Participants preferred side dishes with 2-3 colors.
- Visual appeal played a decisive role in their choices.
This demonstrates how plating’s ability to enhance appearance extends beyond industrial applications to everyday consumer experiences.
Extending product lifespan
By protecting materials from corrosion, wear, and chemical exposure, plating extends the lifespan of products. This reduces the need for frequent replacements, saving costs and resources. Industries such as automotive and electronics rely on plating to ensure their products remain functional and reliable over time.
Types of plating

Plating methods vary based on the process and desired outcomes. Each type offers unique advantages, making them suitable for specific applications across industries.
Electroplating
Electroplating uses an electric current to deposit a thin layer of metal onto a substrate. This process enhances surface properties such as corrosion resistance, durability, and aesthetic appeal. Industries like automotive and electronics rely heavily on electroplating for its ability to produce uniform coatings on complex shapes.
Tip: Electroplating is ideal for applications requiring strong adhesion and precise thickness control.
Key Characteristics:
- Process: Involves immersing the substrate in an electrolyte solution containing metal ions. An electric current facilitates the deposition of metal onto the surface.
- Performance: Provides excellent adhesion and uniformity, making it suitable for intricate designs.
| Standard | Description |
|---|---|
| ISO 10289 | Corrosion testing for electroplated coatings. |
| ASTM B571 | Adhesion evaluation for metallic coatings. |
Measurement techniques such as X-ray fluorescence (XRF) and eddy current testing ensure coating thickness meets industry standards. These methods offer high precision, critical for maintaining quality in electroplating.
Electroless plating
Electroless plating employs a chemical reduction process to deposit metal without using electricity. This method is highly versatile, allowing uniform coatings on complex geometries. It is widely used in industries such as aerospace, electronics, and medical devices due to its reliability and performance.
Advantages:
- Uniformity: Coats surfaces evenly, regardless of shape or size.
- Enhanced Properties: Improves wear resistance, hardness, and corrosion resistance.
Applications:
- Electronics: Ensures reliability in miniaturized components.
- Aerospace: Provides durability under extreme conditions.
- Medical Devices: Offers biocompatibility for surgical instruments and implants.
Note: Electroless plating is slower than electroplating but excels in coating complex geometries.
Immersion plating
Immersion plating involves submerging a less noble metal into a solution containing ions of a nobler metal. This process relies on chemical displacement rather than electricity or chemical reduction. While immersion plating is simpler, it may face adhesion challenges compared to other methods.
Characteristics:
- Process: Metal ions in the solution replace atoms on the substrate surface.
- Performance: Slower deposition rates and potential adhesion issues limit its use in high-performance applications.
| Plating Method | Process Description | Performance Characteristics |
|---|---|---|
| Electroplating | Uses electric current to deposit metal. | Strong adhesion and uniform thickness, suitable for complex shapes. |
| Electroless Plating | Chemical reduction deposits metal without electricity. | Good uniformity and versatility, slower deposition rates. |
| Immersion Plating | Submerges a less noble metal in a solution of nobler metal ions. | Slower process with potential adhesion issues. |
Immersion plating is often used for decorative purposes or applications where high durability is not required.
How does the plating process work?
Plating involves a series of carefully executed steps, specialized tools, and precise control of various factors to achieve high-quality results. Understanding the process provides insight into why plating is essential for enhancing material properties and appearance.
Key steps in plating
The plating process consists of three primary stages: pre-treatment, plating, and post-treatment. Each step plays a critical role in ensuring the final coating adheres properly and meets performance standards.
- Pre-treatment: This stage prepares the surface of the workpiece for plating. Methods include:
- Degreasing: Removes oils and contaminants.
- Pickling: Eliminates rust and oxides using acidic solutions.
- Physical cleaning: Uses abrasive techniques to smooth the surface.
- Plating: During this stage, the actual deposition of metal occurs. Electroplating, for example, uses an electrolytic cell to deposit metal ions onto the substrate. The thickness of the coating depends on factors such as current density and plating time.
- Post-treatment: This final stage enhances the durability and appearance of the plated surface. Techniques may include mechanical processes like polishing or chemical treatments such as chromating to improve corrosion resistance.
Tip: Proper pre-treatment ensures strong adhesion, while effective post-treatment enhances the longevity of the coating.
Tools and equipment used
Plating requires specialized tools and equipment to ensure precision and consistency. These tools vary depending on the type of plating process but generally include the following:
- Electrolytic cells: Used in electroplating to facilitate the deposition of metal ions.
- Power supplies: Provide the electric current necessary for electroplating.
- Plating baths: Contain the electrolyte solution and metal ions.
- Cleaning equipment: Includes ultrasonic cleaners and abrasive tools for pre-treatment.
- Measurement devices: Tools like X-ray fluorescence (XRF) and eddy current testers ensure the coating meets thickness and quality standards.
Advanced equipment, such as automated plating systems, enhances efficiency and reduces human error. These systems are particularly valuable in industries requiring high precision, such as electronics and aerospace.
Factors affecting plating quality
Several factors influence the quality of the plating process. These include current density, temperature, and bath composition. Each factor must be carefully controlled to achieve optimal results.
| Key Factors | Influence on Plating Quality |
|---|---|
| Current Density (J) | Significant impact on cathode efficiency and coating thickness |
| Temperature (T) | Affects the electroplating process and coating properties |
| pH Value | Influences the overall performance of the electroplating bath |
For example, increasing current density up to a certain limit improves the properties of nickel-chromium alloy coatings. However, exceeding this limit reduces current efficiency and compromises coating quality.
| Current Density Range | Effect on Coating Quality |
|---|---|
| Below Critical Value | Increased coating quality |
| At Critical Value | Optimal coating properties observed |
| Above Critical Value | Decreased coating quality due to secondary processes |
Temperature also plays a crucial role. Higher temperatures can accelerate the plating process but may lead to uneven coatings if not monitored closely. Maintaining the correct pH value ensures the electrolyte solution remains stable, preventing defects in the final coating.
Note: Consistent monitoring of these factors is essential for achieving high-quality plating results.
Benefits of plating
Plating offers numerous advantages that enhance the performance, longevity, and appearance of materials. These benefits make it an essential process across industries.
Corrosion resistance
Plating significantly improves an object’s ability to withstand harsh environments. Creating a protective barrier prevents moisture, chemicals, and other corrosive agents from damaging the underlying material. Different metals provide varying levels of corrosion resistance:
- Gold delivers exceptional resistance and conductivity, making it ideal for high-reliability electronic connectors.
- Nickel combines durability with corrosion resistance, often serving as a robust underlayer.
- Zinc offers sacrificial corrosion protection, shielding the base metal and extending its lifespan.
These properties make plating indispensable in industries like automotive and aerospace, where materials face constant exposure to challenging conditions.
Tip: Selecting the right plating material ensures optimal protection against corrosion, tailored to specific applications.
Enhanced durability
Plating enhances the durability of materials by increasing their resistance to wear, abrasion, and impact. This improvement is particularly valuable in high-stress environments. For example, nickel plating adds surface hardness, reducing the risk of scratches and dents. Similarly, chrome plating provides a tough, wear-resistant finish, often used in machinery and tools.
By reinforcing the surface, plating reduces maintenance needs and extends the operational life of products. This durability translates to cost savings and improved reliability, especially in industries like manufacturing and construction.
Improved appearance
Plating transforms the visual appeal of objects by adding a polished, attractive finish. It can create a shiny, reflective surface or a matte texture, depending on the desired aesthetic. Gold and silver plating, for instance, elevate the elegance of jewelry and decorative items. In industrial applications, chrome plating enhances the sleek appearance of automotive parts.
A well-plated surface not only looks appealing but also conveys a sense of quality and craftsmanship. This makes plating a popular choice in consumer goods, where appearance plays a critical role in purchasing decisions.
Note: Plating combines functionality with aesthetics, making it a versatile solution for both industrial and decorative purposes.
Plating vs. coating
Plating and coating are two distinct surface treatment methods, each offering unique advantages depending on the application. Understanding their differences helps in selecting the most suitable option for specific needs.
Key differences
Plating involves applying a thin layer of metal to a substrate, typically through electrochemical or chemical processes. This method enhances durability, wear resistance, and adhesion by bonding at a molecular level. Coating, on the other hand, uses materials like powder, epoxy, or paint to create a protective barrier on the surface. Its performance depends on the chemical composition and application technique.
Key distinctions include:
- Durability: Plating generally provides a longer lifespan under consistent conditions. Coatings may require periodic reapplication, especially in harsh environments.
- Adhesion: Plating forms a strong bond with the substrate, while coatings adhere less firmly but offer easier maintenance.
- Environmental Resistance: Coatings can be engineered for specific conditions, such as UV or chemical exposure, but their resilience varies significantly.
Plating excels in applications requiring consistent wear resistance and hardness, while coatings offer versatility in protective properties.
Tip: For applications demanding high precision and durability, plating is often the preferred choice.
When to choose plating over coating
Plating is ideal for industries where durability, corrosion resistance, and precise thickness are critical. For example, ASTM B633 specifies electrodeposited zinc plating for steel, copper, and brass parts. This standard requires a minimum thickness of 5 µm and mandates passing a salt spray test to ensure corrosion protection. Such rigorous guidelines highlight plating’s reliability in demanding environments.
| Specification | Details |
|---|---|
| Plating Thickness | Minimum of .0002 inch (5 µm) on significant surfaces. |
| Testing Requirements | Must pass 72 hours of salt spray testing with no white corrosion. |
| Industry Applications | Automotive, aerospace, and electronics for enhanced durability. |
Plating is particularly advantageous in high-stress applications, such as automotive components exposed to friction and moisture. Its molecular-level adhesion ensures long-lasting performance, making it superior to coatings in these scenarios. Coatings, while versatile, may not meet the stringent durability and adhesion requirements of such industries.
Note: Choose plating when precision, longevity, and resistance to extreme conditions are non-negotiable.
Plating is a transformative process that enhances the functionality and appearance of materials by applying a thin layer of metal to their surfaces. Its importance lies in improving durability, corrosion resistance, and aesthetic appeal, making it indispensable across industries. From electroplating to immersion plating, each method offers unique advantages tailored to specific applications.
Industries such as automotive, electronics, and jewelry rely heavily on plating to achieve superior performance and visual appeal. For example, automotive parts benefit from enhanced durability, while jewelry gains protection against tarnishing. The table below highlights the diverse applications and benefits of plating across various sectors:
| Industry/Application | Benefits of Plating | Examples of Plating Types |
|---|---|---|
| Automotive | Enhances durability and aesthetics of parts | Electroplating (copper, nickel) |
| Jewelry | Improves appearance and prevents tarnishing | Silver electroplating |
| Telecommunications | Provides superior conductivity for connectors | Palladium and gold plating |
| General Manufacturing | Cost-effective alternative for hardware products with excellent corrosion resistance | Tin electroplating |
| Food Industry | Protects against contamination and improves aesthetics | Coating for various components |
Plating’s versatility and effectiveness make it a cornerstone in modern manufacturing. Its ability to combine functionality with aesthetics ensures its continued relevance in industries that demand high-quality materials and finishes.
FAQ
What materials can be plated?
Plating works on various materials, including metals like steel, aluminum, and copper. Non-metallic surfaces, such as plastics, can also undergo plating after special preparation. The choice of material depends on the application and desired properties.
Is plating environmentally friendly?
Plating can be environmentally friendly when performed with proper waste management and eco-friendly chemicals. Modern techniques focus on reducing hazardous waste and recycling materials, making the process more sustainable.
Tip: Choose plating services that comply with environmental regulations for a greener approach.
How long does plating last?
The lifespan of plating depends on factors like material type, coating thickness, and environmental exposure. High-quality plating can last several years, especially when maintained properly. Regular inspections and cleaning help extend its durability.
Can plating be repaired?
Yes, damaged plating can often be repaired through re-plating or touch-up processes. The repair method depends on the extent of the damage and the type of plating. Consulting a professional ensures the best results.
What industries benefit most from plating?
Industries like automotive, electronics, aerospace, and jewelry benefit significantly from plating. It enhances durability, corrosion resistance, and appearance, making it essential for high-performance and decorative applications.
Note: Plating is also widely used in medical devices and telecommunications for its reliability and precision.
