
Tin plating is a process of coating a base metal with a thin layer of tin to improve its properties and performance. This technique plays a crucial role in industries that require enhanced durability, corrosion resistance, and electrical conductivity. For example, the global tin metal market, driven by applications in electronics and packaging, is projected to grow significantly, reaching USD 10.5 billion by 2032. Industries such as automotive and electronics rely heavily on tin plating, as it ensures product longevity and optimal functionality. This growing demand highlights the significance of tin plating in contemporary manufacturing.
Key Takeaways
- Tin plating makes metals stronger and last longer. It stops rust, helps electricity flow, and makes soldering easier.
- There are two main ways to do tin plating: electroplating and hot-dipping. Each works best for different uses.
- Tin plating is cheap because tin is easy to find. This makes it popular in electronics and food packaging industries.
- Cleaning the surface well and checking quality are very important. These steps help tin plating work better and last longer.
- Tin plating is very useful in many industries like cars, electronics, and packaging. It makes products safer and work better.
Tin Plating Basics
Definition and Purpose
Tin plating involves coating a base metal with a thin layer of tin to enhance its properties. This process serves multiple purposes, including improving corrosion resistance, electrical conductivity, and solderability. Tin plating protects metals like iron from rusting by forming a barrier against moisture and oxygen. It also ensures the purity of substances, such as water and beverages, when used in piping and valves. Furthermore, tin plating plays a critical role in manufacturing float plate-glass, where it acts as a base material during production.
The industry has established standards to define the purpose and quality of tin plating. For instance, ASTM B545 and MIL-T-10727 specify minimum thicknesses and service conditions for various applications. These standards ensure that tin plating meets the requirements of different industries, from mild service environments to soldered articles.
| Standard | Class/Type | Minimum Thickness (inches) | Service Conditions |
|---|---|---|---|
| ASTM B545 | A | 0.0001 | Mild service, shielded from atmosphere |
| ASTM B545 | B | 0.0002 | Mild service, exposed significant surface |
| ASTM B545 | C | 0.00032 (nonferrous) | Moderate service conditions |
| MIL-T-10727 | Type I | 0.0001-0.00025 | Tin flashing for soldered articles |
| AMS 2408 | – | 0.0001-0.0003 | General tin plating specifications |
Types of Tin Plating
Tin plating can be categorized into two primary types: electroplating and hot-dipping. Each method offers unique advantages and is suited for specific applications.
- Electroplating: This method involves immersing the base metal in an electrolytic solution. Two electrodes, a cathode (negative charge) and an anode (positive charge), are placed in the solution. When an electrical current passes through, positive ions of tin adhere to the base metal, forming a uniform coating. Electroplating is widely used for its precision and ability to control the thickness of the tin layer.
- Hot-Dipping: In this process, the base metal is dipped into molten tin. The high temperature ensures a strong bond between the tin and the base metal. Hot-dipping is ideal for applications requiring thicker coatings and enhanced durability.
These methods allow manufacturers to tailor the tin plating process to meet specific needs, whether for aesthetic appeal, functional performance, or cost efficiency.
| Characteristic | Tin Plating | Silver Plating |
|---|---|---|
| Corrosion Resistance | Robust, forms a protective oxide layer | Less prone to oxidation but can tarnish |
| Electrical Conductivity | Good, suitable for electronics | Superior, best for maximum efficiency |
| Cost | More budget-friendly, ideal for mass production | More expensive, justified for superior applications |
| Aesthetic Appeal | Duller appearance, less durable | Mirror-like finish, enhanced durability |
| Solderability | Highly solderable, ideal for electronics | Less solderable compared to tin |
Common Applications
Tin plating finds extensive use across various industries due to its versatility and cost-effectiveness. Some of the most common applications include:
- Food and Beverage Packaging: Tin-plated steel is widely used for canned foods and beverages. It prevents contamination and ensures product safety. The global demand for tinplate in this sector is growing at an annual rate of 3%.
- Industrial Applications: Tin plating is essential for manufacturing paint cans, which prevent leakage and maintain product quality.
- Aerosol Cans: Tin-plated cans withstand high pressure, making them suitable for aerosol products.
- Household and Consumer Goods: Durable and attractive tin-plated containers, such as biscuit tins, are popular among consumers.
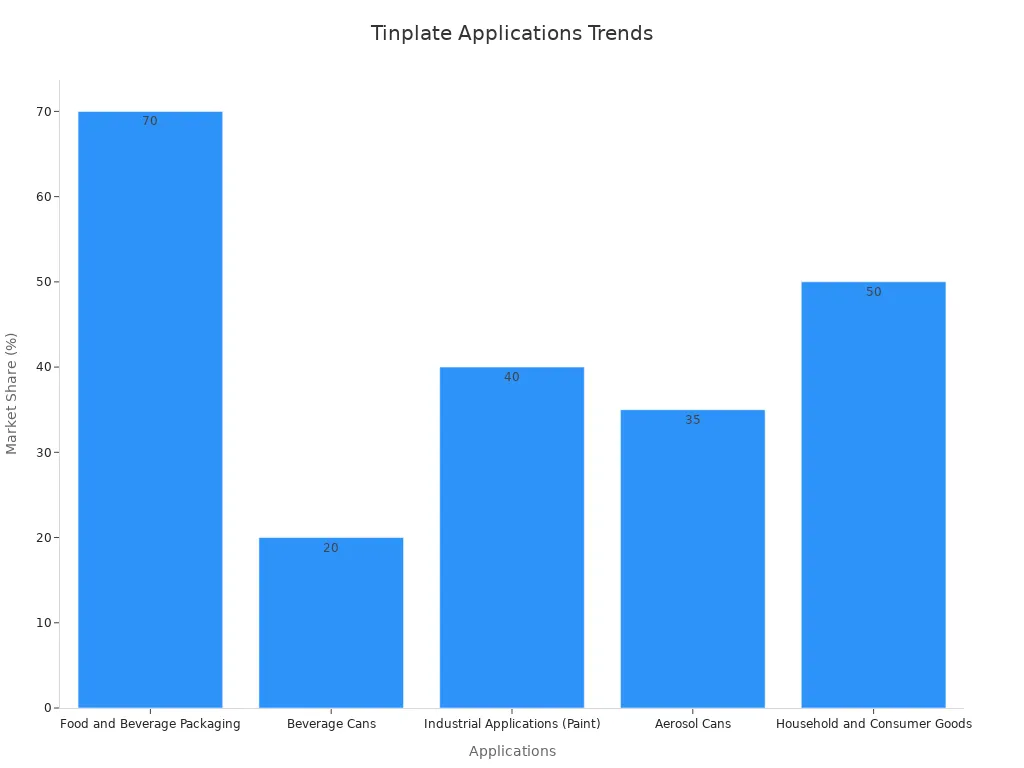
These applications highlight the importance of tin plating in ensuring product longevity, safety, and functionality across diverse industries.
Tin Plating Process

Electroplating
Electroplating is one of the most precise methods used in tin plating. This process involves immersing the base metal in an electrolytic solution containing tin ions. A direct current is applied, causing the tin ions to deposit onto the surface of the base metal. This method allows manufacturers to control the thickness of the tin layer with high accuracy, making it ideal for applications requiring uniform coatings.
The effectiveness of electroplating can be measured through various outcomes. For instance, plating current efficiency is calculated using an electrochemical quartz crystal microbalance (EQCM). Corrosion resistance is evaluated by exposing the plated material to a 0.5M Na₂SO₄ solution at open circuit potential (OCP). The stability of the OCP indicates the primary reactions occurring during the process, while the mass loss rate during corrosion provides insights into the durability of the coating.
| Measured Outcome | Description |
|---|---|
| Plating Current Efficiency | Calculated using an electrochemical quartz crystal microbalance (EQCM). |
| Corrosion Rate | Measured during exposure to a 0.5M Na₂SO₄ solution at open circuit potential (OCP). |
| Stability of Open Circuit Potential | Indicates the major reaction occurring at OCP, specifically the dissolution of zinc. |
| Mass Loss Rate | Steady state mass loss rate of about 2.42 x 10⁻³ μg/cm²-sec (1.56 x 10⁻² μg/in.²-sec) during corrosion. |
Electroplating is widely used in the electronics industry due to its ability to produce thin, conductive layers of tin. This method also ensures excellent solderability, making it a preferred choice for circuit boards and connectors.
Hot-Dipping
Hot-dipping is another popular method for tin plating, known for its simplicity and cost-effectiveness. In this process, the base metal is immersed in molten tin at high temperatures, typically between 260°C and 280°C. This temperature range ensures a strong bond between the tin and the base metal while maintaining the micro-hardness of the coating at 95% of its original value.
The hot-dipping process involves several steps, including alkaline cleaning, water scrubbing, and drying. These steps ensure a uniform coating morphology and desirable mechanical properties. Compared to electroplating, hot-dipping is more environmentally friendly and cost-efficient. It also offers excellent solderability and minimal pollution, making it a promising technique for future applications.
- Advantages of Hot-Dipping:
- Cost-effective and environmentally friendly.
- Produces a uniform and durable coating.
- Simple procedures with minimal pollution.
- Optimal for applications requiring thicker tin layers.
Hot-dipping is commonly used in industries such as food packaging and construction, where durability and corrosion resistance are critical.
Surface Preparation and Finishing
Surface preparation is a crucial step in the tin plating process. It ensures that the base metal is clean and free from contaminants, which could affect the adhesion and quality of the tin coating. Common preparation techniques include degreasing, acid pickling, and abrasive cleaning. These methods remove oils, oxides, and other impurities from the surface.
After plating, finishing processes enhance the appearance and functionality of the coated material. Finishing may involve polishing, passivation, or applying a protective topcoat. Quality assurance measures are essential during this stage to ensure the coating meets design specifications. Thickness is measured using tools like micrometers or x-ray fluorescence. Adhesion tests, such as bend or tape pull tests, confirm the bond between the tin layer and the substrate. Visual inspections and microscopic analyses identify any defects or imperfections.
| Quality Assurance Measure | Description |
|---|---|
| Thickness | Measured to confirm it meets design specifications; checked before and after plating using micrometers or x-ray fluorescence. |
| Adhesion | Confirms proper bonding between substrate and plated layer; tested using methods like bend, chisel, or tape pull tests. |
| Appearance | Visually inspected for defects; microscopic imperfections analyzed using SEM or profilometers. |
Proper surface preparation and finishing not only improve the durability of the tin plating but also enhance its aesthetic appeal and performance in demanding applications.
Benefits of Tin Plating

Corrosion Resistance
Tin plating offers exceptional corrosion resistance, making it a preferred choice for industries requiring durable and long-lasting components. The tin layer acts as a protective barrier, shielding the base metal from moisture, oxygen, and other corrosive elements. This property is particularly valuable in environments prone to high humidity or exposure to chemicals. For instance, in the food processing and packaging industry, tin plating ensures the safety and freshness of products by preventing contamination and maintaining the integrity of containers.
Testing results further highlight its effectiveness. According to ASTM B117 salt spray tests, thin tin coatings can withstand 200 to 500 hours of exposure, depending on factors such as coating thickness and the presence of a passivation layer. This durability underscores the reliability of tin plating in extending the lifespan of components across various applications.
| Corrosion Resistance (Salt Spray) | Hours | Testing Method | Factors Influencing Performance |
|---|---|---|---|
| Thin Coatings | 200-500 | ASTM B117 | Coating thickness, passivation layer, environment |
Electrical Conductivity
Tin plating enhances electrical conductivity, a critical requirement in the electronics and telecommunications industries. The tin layer ensures efficient current flow, reducing energy loss and improving the performance of electrical systems. This property makes tin plating indispensable for circuit boards, connectors, and other electronic components. In telecommunications, it ensures reliable data transmission by protecting surfaces from corrosion, which could otherwise disrupt signal quality.
The automotive industry also benefits from this property, as tin plating improves the performance of electrical systems in vehicles. By ensuring consistent conductivity, it contributes to the reliability and efficiency of modern automotive designs.
Solderability
Solderability is another significant advantage of tin plating. The tin layer provides a smooth and uniform surface, facilitating strong and reliable solder joints. This property is essential in the manufacturing of electronic devices, where precise and durable connections are critical. Tin plating also reduces the risk of oxidation during the soldering process, ensuring consistent results.
Industries such as aerospace rely on this feature to protect critical components and maintain reliability in extreme environments. The combination of excellent solderability and corrosion resistance makes tin plating a versatile solution for demanding applications.
Tip: Tin plating not only enhances functionality but also provides cost-effective surface protection, making it a valuable choice across multiple industries.
Cost-Effectiveness
Tin plating stands out as a cost-effective solution in surface finishing due to the abundance and affordability of tin. Compared to precious metals like gold or platinum, tin offers a significantly lower cost while delivering excellent performance. Its unique properties, such as corrosion resistance and solderability, further enhance its value across multiple industries.
The electronics and electrical manufacturing sectors heavily rely on tin plating for its economic advantages. Tin’s solderability ensures reliable connections in components like printed circuit boards (PCBs) and connectors. This makes it indispensable for consumer electronics, where cost efficiency is critical. The global PCB market, valued at $78 billion in 2023, depends on tin plating for over 65% of hardware used in consumer electronics. This reliance highlights tin plating’s role in reducing production costs while maintaining high-quality standards.
- Key reasons for tin plating’s cost-effectiveness:
- Tin is widely available, making it more affordable than rare metals.
- Its durability reduces the need for frequent replacements, lowering long-term costs.
- The process is versatile, accommodating various applications without significant expense.
Tin plating also benefits industries like food packaging and automotive manufacturing. Its ability to provide a protective barrier at a low cost makes it an ideal choice for mass production. Manufacturers can achieve both functionality and affordability, ensuring competitive pricing for their products.
Note: Tin plating combines affordability with performance, making it a preferred choice for industries seeking cost-efficient solutions without compromising quality.
Quality Control in Tin Plating
Inspection Techniques
Effective quality control in tin plating begins with robust inspection techniques. These methods ensure the coating adheres to specifications and performs as intended. Commonly used techniques include visual inspections, adhesion tests, and corrosion resistance evaluations. Visual inspections identify surface defects such as cracks, discoloration, or uneven coatings. Adhesion tests, like the bend test and pull-off test, assess the bond strength between the tin layer and the substrate. For corrosion resistance, salt spray testing and cyclic corrosion testing simulate environmental conditions to evaluate the durability of the plating.
| Test Method | Description |
|---|---|
| Bend Test | Evaluates adhesion by bending the substrate; good adhesion is indicated if the coating does not crack. |
| Pull-off Test | Measures the force required to detach the coating, providing a quantitative assessment of adhesion. |
| Salt Spray Test | Exposes the specimen to salt mist to assess corrosion resistance; rust presence indicates durability. |
| Cyclic Corrosion Testing | Simulates various environmental conditions to evaluate long-term performance of the metal finish. |
These inspection techniques provide manufacturers with critical data to maintain high-quality standards in tin plating.
Testing for Solderability
Solderability testing ensures that tin-plated surfaces can form reliable solder joints, a crucial requirement in electronics and other industries. ASTM B678 serves as the standard test method for evaluating solderability. It verifies that solder can wet and spread over the surface, ensuring strong connections. The wetting balance test is a widely used quantitative method. It measures the forces acting on a specimen immersed in molten solder, providing insights into the coating’s performance.
- Key aspects of solderability testing:
- Flux Used: Nonactivated rosin in alcohol ensures better discrimination during testing.
- Coating Defects: Identifies issues like porosity or contamination that may hinder solderability.
- Compliance: Adheres to international standards like IEC 60068-2-69 and J-STD-002.
These tests confirm the effectiveness of tin plating in applications requiring precise and durable solder joints.
Addressing Defects
Defects in tin plating can compromise performance and durability. Common issues include uneven coatings, porosity, and oxidation. Addressing these defects requires a combination of preventive measures and corrective actions. Surface preparation plays a vital role in minimizing defects. Proper cleaning and degreasing remove contaminants that could affect adhesion. During the plating process, maintaining consistent parameters such as temperature and current density ensures uniform coatings.
Post-plating inspections help identify defects early. For example, dry heat aging and steam aging tests assess the coating’s resistance to oxidation and intermetallic growth. Selective electrochemical reduction analysis (SERA) evaluates solderability, ensuring the coating meets industry standards.
| Testing Method | Description |
|---|---|
| Dry Heat Aging | Conducted at 155°C for 4-8 hours to assess oxidation and intermetallic growth. |
| Steam Aging Testing | Not suitable for non-fused finishes like OSPs and immersion tin. |
| SERA Testing | Selective electrochemical reduction analysis for solderability evaluation. |
By implementing these strategies, manufacturers can enhance the quality and reliability of tin plating, ensuring it meets the demands of various applications.
Tin plating remains a cornerstone of modern manufacturing due to its versatility and performance. Its exceptional properties, such as corrosion resistance, solderability, and electrical conductivity, make it indispensable across industries. For example:
- Automotive applications benefit from reliable electrical connections and superior durability.
- Electronics leverage tin’s conductivity for circuit boards and connectors.
- Packaging ensures food safety and extended shelf life.
| Application Sector | Key Benefits | Market Growth Rate |
|---|---|---|
| Automotive | Superior corrosion resistance, solderability | Estimated CAGR of 5% |
| Electronics | Excellent conductivity, reliability | |
| Packaging | Enhances shelf life, food safety |
By enhancing durability and functionality, tin plating continues to drive innovation and efficiency in diverse applications.
FAQ
What is the difference between electroplating and hot-dipping in tin plating?
Electroplating uses an electrolytic solution and electrical current to deposit tin onto a base metal, offering precise control over coating thickness. Hot-dipping involves immersing the metal in molten tin, creating a thicker, more durable layer. Each method suits different applications based on required coating properties.
How does tin plating improve corrosion resistance?
Tin plating forms a protective barrier that prevents moisture and oxygen from reaching the base metal. This barrier reduces the risk of rust and chemical reactions, making it ideal for environments with high humidity or exposure to corrosive substances.
Can tin plating be applied to all metals?
Tin plating works best on metals like steel, copper, and brass. Surface preparation ensures proper adhesion. However, some metals may require additional treatments or primers to achieve optimal results.
Is tin plating environmentally friendly?
Tin plating is considered environmentally friendly, especially when using hot-dipping. Tin is non-toxic and recyclable, and modern plating processes minimize waste and pollution. Industries increasingly adopt eco-friendly practices to reduce environmental impact.
What industries benefit most from tin plating?
Industries like electronics, automotive, food packaging, and aerospace benefit significantly. Tin plating enhances electrical conductivity, corrosion resistance, and solderability, making it indispensable for circuit boards, connectors, food cans, and critical aerospace components.
Tip: Choose the tin plating method that aligns with your industry’s specific needs for durability, cost, and performance.
