1.Understanding the Importance of Material Magnetism
Magnetism is a fundamental property of matter that influences a wide range of scientific and industrial applications. Understanding the magnetism of materials is crucial for fields such as electronics, medicine, and manufacturing. Lead is one such material that has sparked curiosity due to its extensive industrial uses. In this article, we will explore the magnetism of lead, delving into its electron structure, the impact of impurities, and its behavior in magnetic fields. By the end, you will have a comprehensive understanding of why lead is classified as a diamagnetic material and the significance of this property.
2.Does Lead Have Magnetism?
Lead is typically considered non-magnetic because the magnetic moments of its electrons cancel each other out. It exhibits diamagnetism, meaning it is repelled by magnetic fields. This magnetic behavior arises from the filled 6s and 6p orbitals in the atomic structure of lead. These filled orbitals produce paired electrons with opposing magnetic moments. The magnetism of lead is weak, manifesting as a lack of response to external magnets and the absence of permanent magnetism.
Types of Magnetism: Materials can exhibit various types of magnetism: ferromagnetism, paramagnetism, and diamagnetism.
For example:
- Ferromagnetic materials like iron, cobalt, nickel, etc., possess strong magnetism and can be permanently magnetized.
- Paramagnetic materials exhibit weak attraction to magnetic fields and do not retain magnetism once the external magnetic field is removed.
- Diamagnetic materials are repelled by magnetic fields and do not retain any magnetization strength.
Lead falls into the diamagnetic category, meaning it exhibits weak repulsion to magnetic fields.
3.How the electronic structure of lead affects nonmagnetic properties
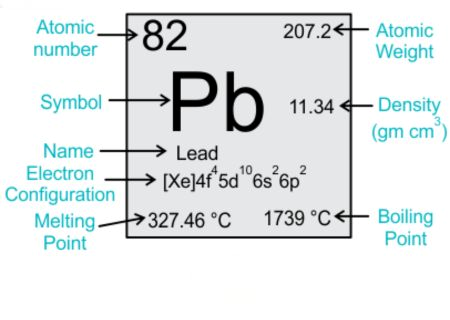
Electronic Configuration of Lead (Pb)
The magnetic properties of any material are deeply rooted in its atomic structure, especially its electron configuration. Lead has an atomic number of 82 and an electron configuration of [Xe] 4f^14 5d^10 6s^2 6p^2.
The inner shells (highest 4f and 5d levels) are filled with electrons.
The outermost shell (6s and 6p orbitals) contains 4 electrons.
This electron structure results in lead lacking magnetism. Magnetism arises from the motion of electrons and their arrangement of magnetic moments within a material. In ferromagnetic materials, unpaired electrons in the outer shell align their spins in the same direction, resulting in a strong magnetic field. However, in lead, the electrons are paired, and their magnetic moments cancel each other out. This leads to no net magnetic moment.
Quantum mechanics further explains this phenomenon through the concepts of electron spin and orbital angular momentum. In diamagnetic materials like lead, the electrons in filled shells produce a small magnetic field opposite to any external magnetic field, resulting in repulsion. The inherent property of electron pairing and filled shell explains why lead exhibits diamagnetic behavior.
4. How Impurities Affect the Magnetism of Lead
While pure lead displays diamagnetism, significant changes occur in the magnetism of lead when ferromagnetic materials are added to lead alloys.
Impurities introduce localized regions with different electron configurations, which in some cases may result in slight paramagnetic or even weak ferromagnetic behavior.
Common impurities in lead include elements such as tin, antimony, and bismuth. Each of these impurities affects the magnetism of lead in different ways:
Tin (Sn): Often added to improve the mechanical properties of lead. Tin itself is diamagnetic, so it does not significantly alter the overall diamagnetic properties of lead.
Antimony (Sb): Used for hardening lead, antimony is another diamagnetic element. However, high concentrations may cause local distortion in the electron cloud, potentially affecting magnetism slightly.
Bismuth (Bi): Known for its strong diamagnetism, bismuth can enhance the diamagnetic behavior of lead when used as an impurity.
Experimental data indicate that the content and type of impurities can have measurable effects. For example, small amounts of magnetic impurities (such as iron or cobalt) introduce paramagnetic behavior. Studies involving lead alloys have demonstrated these effects, highlighting the importance of controlling impurity levels in applications requiring specific magnetic properties.
5. Diamagnetism of Lead
Diamagnetism is a form of magnetism exhibited by all materials to some extent. However, in diamagnetic materials like lead, this characteristic predominates. Diamagnetic materials produce an induced magnetic field opposite to the externally applied magnetic field and are repelled by the applied magnetic field.
The primary characteristic of diamagnetic materials is that they do not retain magnetization strength when there is no external magnetic field. When exposed to a magnetic field, lead generates a weak magnetic moment in the opposite direction, resulting in its repulsion. This effect is subtle and requires sensitive instruments to detect, unlike the strong attraction seen in ferromagnetic materials.
6. The Effect of Magnets on Lead
Compared to materials with stronger magnetism, the interaction between lead and magnetic fields is minimal. Magnetization, which quantifies the degree to which a material becomes magnetized in an applied magnetic field, is a measure of this interaction. For diamagnetic materials, magnetization is negative, indicating an induced magnetic field opposite to the applied magnetic field. For lead, its magnetization is approximately -1.8 x 10^-5 (SI units). This value is typical for weak diamagnetic materials and is much lower than that of ferromagnetic materials.
6.1. Wires Not Attracted by Magnets
Like any other form of lead, wires are not attracted by magnets. When a magnet is brought near a lead wire, the wire experiences a weak repulsive force due to its diamagnetic nature. This repulsion is usually too weak to observe without sensitive equipment but is a fundamental characteristic of diamagnetic materials.
In practical applications, wires can be used in strong magnetic field environments without significant magnetic force. The characteristic of minimizing magnetic interference is advantageous in certain industrial and electronic applications.
6.2. Magnetization Susceptibility of Lead
Magnetization susceptibility is a measure of the degree of magnetization of a material in an externally applied magnetic field. For lead, the susceptibility is negative, indicating that it will produce a magnetic moment opposite to the direction of the applied magnetic field.
7. Industrial Technological Applications of Lead’s Diamagnetism
Understanding these properties of lead, its diamagnetism finds widespread applications in industrial technology.
7.1. Industrial Applications
7.1.1 Radiation Shielding
The high density and atomic number of lead make it an excellent radiation shielding material. Its diamagnetic properties are advantageous in strong magnetic field environments, such as medical imaging facilities and nuclear power plants. The weak repulsive force to magnetic fields ensures that lead shielding remains stable and unaffected by magnetic forces, maintaining its effectiveness in radiation protection.
7.1.2 Cable Sheathing
Lead is commonly used as cable sheathing, especially in environments with electromagnetic interference (EMI). The diamagnetic nature of lead helps reduce the impact of external magnetic fields on cables, ensuring signal integrity. This application is crucial in industries such as telecommunications, aerospace, and military, where reliable data transmission is essential.
7.1.3 Battery Manufacturing
Lead-acid batteries are widely used in automotive and backup power applications. The diamagnetic properties of lead ensure that the battery’s performance is not affected by external magnetic fields. This stability is crucial for maintaining consistent power output and lifespan under various operating conditions.
7.2. Technological Applications
7.2.1 Superconductors
In superconducting applications, materials are cooled to extremely low temperatures to exhibit zero resistance. Lead’s diamagnetism contributes to creating an environment with minimal magnetic interference in superconducting research and technology, allowing superconductors to maintain their unique performance without disturbance.
7.2.2 Magnetic Resonance Imaging (MRI)
Lead is used to shield MRI rooms to prevent external electromagnetic field interference with the imaging process. Its diamagnetic properties ensure
that lead shielding remains unmagnetized, providing stable and effective protection. This application is critical for obtaining high-quality medical images and accurate diagnoses.
7.2.3 Precision Instruments
Instruments used for precise measurements, such as atomic clocks and quantum computing devices, require environments free from magnetic interference. Lead’s diamagnetic properties make it an ideal material for constructing parts and shields in these instruments, ensuring the accuracy and reliability of measurements and calculations.
7.3. Safety Considerations
7.3.1 Electromagnetic Compatibility (EMC)
Electromagnetic compatibility is crucial for preventing mutual interference between electronic devices. The diamagnetic properties of lead can provide stable and reliable electromagnetic field shielding, aiding in EMC. This application is essential in consumer electronics, automotive systems, and medical devices, where interference could lead to malfunctions or safety hazards.
7.3.2 Protective Equipment
Lead-based protective equipment such as aprons and gloves are used in environments with high radiation levels. The diamagnetic properties of lead ensure that these protective items do not interact with magnetic fields, maintaining their integrity and effectiveness. This application is critical for the safety of workers in medical, nuclear, and industrial environments.
8. Conclusion
In conclusion, lead is a diamagnetic material, exhibiting weak repulsion in the presence of a magnetic field. This behavior is a result of its electron structure, where paired electrons produce opposing magnetic fields, canceling out any external influences. While impurities may slightly affect the magnetism of lead, diamagnetism overall remains predominant. The low magnetization susceptibility of lead makes it suitable for applications where minimal interaction with magnetic fields is desired. Understanding these properties helps make wiser use of lead in various industrial and technological environments.