
Tungsten is magnetic, but not in the way you might think. This metal has the highest melting point of any metal at 3422°C (6192°F), along with remarkable properties that set it apart in metallurgy. Its incredible density reaches 19.25 g/cm³, making it one of the densest metals you can find, yet tungsten won’t stick to your refrigerator like iron or nickel.
The metal’s position on the periodic table creates an interesting situation regarding its magnetic behavior. Scientists classify it as a paramagnetic material, which means it shows a weak positive susceptibility to magnetic fields. Tungsten’s paramagnetic nature comes from its unpaired electrons, which create a subtle attraction that you’d barely notice in daily life. Pure tungsten can be considered magnetic or nonmagnetic based on your definition, but its attraction is so weak that tungsten rings stay clear of magnetic surfaces. The metal’s magnetic properties can change based on its purity and any impurities present. This piece explores the science behind tungsten’s unique magnetic behavior and how it matters to industries of all types.
Understanding Tungsten’s Atomic and Physical Structure
Understanding tungsten’s unique magnetic properties requires examining its basic atomic and physical structure. The way electrons and atoms arrange themselves are the foundations of how tungsten behaves on a larger scale.
Electron Configuration and Atomic Number of Tungsten
Tungsten sits at atomic number 74 on the periodic table among transition metals. Its complex electron configuration reads [Xe]4f¹⁴5d⁴6s². The metal has four unpaired electrons in its 5d orbital. These unpaired electrons create magnetic moments that shape tungsten’s magnetic behavior.
Unpaired electrons make tungsten paramagnetic instead of completely nonmagnetic. The metal’s electronic structure creates a perfect balance – it responds to external magnetic fields but won’t stay magnetized under normal conditions.
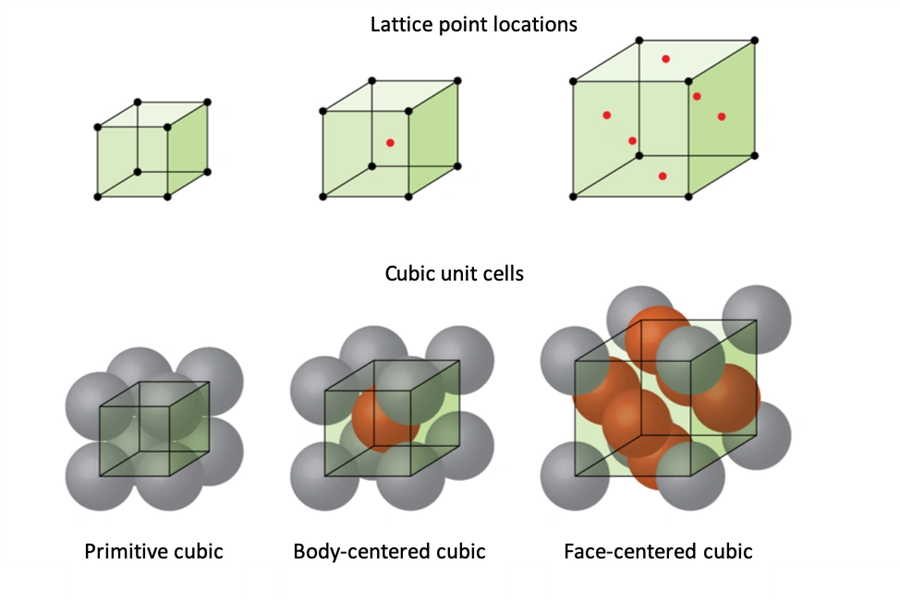
Body-Centered Cubic Crystal Structure
Metallurgists know that tungsten atoms form a body-centered cubic (BCC) crystal structure. This arrangement shows:
- One tungsten atom at each corner of a cube
- One additional tungsten atom at the center of the cube
- Strong metallic bonding between atoms
The crystalline arrangement gives tungsten incredible stability and leads to its high melting point. The BCC structure affects how electron spins interact throughout the material, which influences its magnetic behavior.
Tight packing and strong bonds between atoms limit how magnetic moments line up. This explains why tungsten doesn’t show ferromagnetism like iron, even though it has unpaired electrons. The crystal structure blocks magnetic domains from forming, which would otherwise create stronger magnetic properties.
Tungsten Periodic Table Placement and Group 6 Properties
Tungsten belongs to Group 6 (formerly VIB) of the periodic table alongside chromium and molybdenum. Its family position gives it special traits:
- High melting and boiling points
- Excellent thermal conductivity
- Most important hardness and density
- Moderate electrical conductivity
- Resistance to corrosion
As a d-block element with partially filled d-orbitals, tungsten shows paramagnetic behavior. All Group 6 elements display paramagnetism due to their electron setup, though tungsten’s is weaker than other transition metals.
Comparing tungsten with nearby elements shows why its periodic placement matters. Elements to the right usually have more paired electrons, making them less paramagnetic or even diamagnetic. Elements to the left tend to show stronger magnetic properties.
This placement puts tungsten in a magnetic sweet spot – neither strongly magnetic like iron nor nonmagnetic like zinc. Its atomic structure creates just enough unpaired electrons for weak paramagnetic behavior without becoming ferromagnetic.
Types of Magnetism and Where Tungsten Fits
Materials react differently to magnetic fields because of their atomic structure. This reaction shapes a material’s magnetic properties and decides how we can use it in engineering and manufacturing.
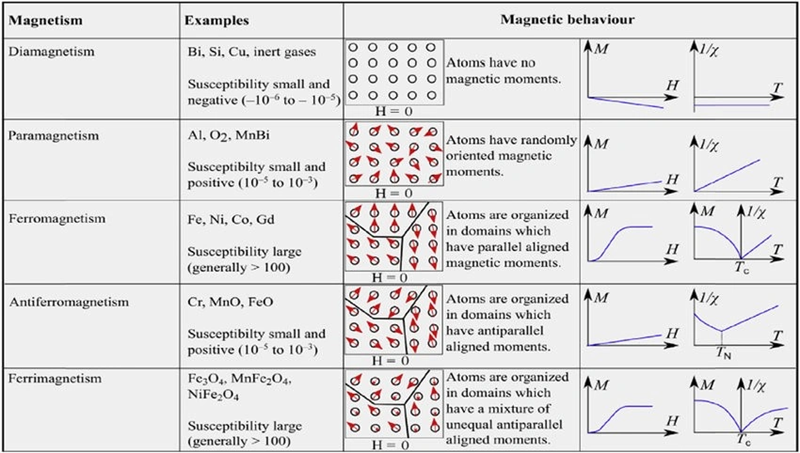
Ferromagnetism vs Paramagnetism vs Diamagnetism
Magnetic behavior falls into three main types. Each type shows unique reactions when materials meet magnetic fields:
Ferromagnetic materials show the strongest magnetic response we commonly see. Iron, cobalt, and nickel strongly attract to both poles of a magnet. Their magnetic domains line up on their own and stay magnetized even after you remove the external magnetic field. These qualities make ferromagnetic materials perfect for use in permanent magnets and magnetic storage.
Paramagnetic materials show a weak positive pull toward magnetic fields. They have a slight attraction to magnets but lose it right away once you take the field away. This occurs because paramagnetic materials have unpaired electrons that briefly align with an applied magnetic field. Aluminum, platinum, and magnesium represent this group.
Diamagnetic materials push back weakly against magnetic fields. These substances create tiny opposing magnetic fields when exposed to external magnetism. Most elements in the periodic table belong here, including copper, silver, and gold. Their electrons pair up fully, so atoms have no lasting magnetic moment.
Is Tungsten Paramagnetic or Diamagnetic?
Scientists don’t always agree on tungsten’s magnetic properties. Most reliable sources say tungsten is paramagnetic with a magnetic susceptibility of +6.8 × 10⁻⁶ emu/g. This positive number shows it has a weak attraction to magnetic fields, which matches paramagnetic behavior.
Tungsten acts as a paramagnet because of its electron setup ([Xe] 4f¹⁴ 5d⁴ 6s²). It has unpaired electrons in its 5d orbital. These lone electrons create weak magnetic moments that line up briefly with external magnetic fields.
Some sources label tungsten as diamagnetic, saying it pushes back very weakly against magnetic fields. This disagreement likely comes from tungsten’s very weak magnetic response, which sits right between paramagnetic and diamagnetic behavior. The evidence points to tungsten being paramagnetic, though its response is very weak.
In real-world use, many people call tungsten non-magnetic. Its magnetic pull is so small that only sensitive tools can detect it. This quality makes tungsten valuable when you need materials that won’t mess with magnetic fields.
Why Tungsten Is Not Ferromagnetic
Tungsten has unpaired electrons like ferromagnetic materials, but doesn’t act like them. Here’s why:
Tungsten’s crystal structure can’t support magnetic domains. Ferromagnetic materials have domains where 10¹² to 10¹⁵ atomic moments line up parallel and create strong internal magnetic fields. The body-centered cubic structure of tungsten blocks this kind of alignment.
The electron setup in tungsten doesn’t allow for strong exchange interactions that ferromagnetism needs. Even with unpaired electrons in its d-orbital, tungsten’s spin arrangement can’t create the team effort seen in iron, cobalt, and nickel.
Temperature plays a vital role too. Every ferromagnetic material has a Curie temperature. Above this point, heat energy breaks up the alignment of magnetic domains. Tungsten’s electronic structure prevents ferromagnetic ordering even at very low temperatures.
This non-ferromagnetic nature of tungsten matters a lot in precision engineering. Pure tungsten parts work well in strong magnetic fields because they won’t get magnetized or disturb existing magnetic systems. Engineers value this trait in aerospace, defense, and electronics where magnetic interference could hurt performance.
Why Pure Tungsten Is Considered Nonmagnetic

Pure tungsten has a complex relationship with magnetism. While it’s not completely nonmagnetic in technical terms, you wouldn’t notice any magnetic properties in day-to-day use.
Lack of Magnetic Domain Alignment in Tungsten
Pure tungsten’s nonmagnetic nature comes from its atomic structure. The material has unpaired electrons in its outer shells, but its crystal structure stops magnetic domains from forming properly. These domains are areas where magnetic moments point in the same direction, but tungsten just can’t create them effectively.
When magnetic fields act on tungsten, its electrons briefly move toward the field. The electrons go back to their neutral state once you remove the field. This makes tungsten very different from ferromagnetic materials that stay magnetized even after the field goes away.
Tungsten’s body-centered cubic crystal structure limits any chance of magnetic domains forming. This setup creates a stable arrangement where electron paths stay mostly fixed, which prevents the natural alignment needed for ferromagnetism.
Magnetic Susceptibility of Tungsten: +6.8 × 10⁻⁶ emu/g
Magnetic susceptibility shows how much a material magnetizes when exposed to a magnetic field. Tungsten’s magnetic susceptibility sits at about +6.8 × 10⁻⁶ emu/g, which shows its very weak attraction to magnetic fields.
This value puts tungsten in the paramagnetic category, but its effect is so small that it’s treated as nonmagnetic in most real-world uses. Yes, it is worth noting that tungsten’s magnetic permeability is almost the same as free space.
Different sources give slightly different values for tungsten’s magnetic susceptibility:
- Mass magnetic susceptibility: 4.59×10⁻⁹ m³/Kg
- Molar magnetic susceptibility: 8.44×10⁻¹⁰ m³/mol
- Volume magnetic susceptibility: 0.0000884
- +59e-6 cm³/mol
Whatever the specific measurement, all these values show that tungsten barely responds to magnetic fields.
Comparison with Iron and Nickel Magnetic Behavior
Tungsten’s magnetic properties pale next to ferromagnetic metals. Iron, nickel, and cobalt have magnetic susceptibilities around 10³ emu/g—much stronger than tungsten. This huge gap explains why you can make permanent magnets from ferromagnetic materials but not from tungsten.
Temperature plays a big role in magnetic properties. Curie’s law tells us that higher temperatures reduce paramagnetic susceptibility, so tungsten becomes even less magnetic as it heats up.
Ferromagnetic materials have domains with 10¹² to 10¹⁵ aligned atomic moments that create strong magnetic effects. Tungsten has no such domain structure, which results in magnetic properties so weak that you need special equipment to detect them.
This means tungsten parts won’t stick to magnets or become magnetized during production. They won’t interfere with sensitive magnetic equipment either. That’s why aerospace applications that need zero magnetic interference often use tungsten.
Magnetic Behavior of Tungsten Alloys and Carbides
Tungsten alloys and compounds show different magnetic behaviors based on how they’re made and what they contain. Pure tungsten acts differently. These variations create special properties that make tungsten-based materials perfect for specific uses.
Is Tungsten Carbide Magnetic?
Tungsten carbide by itself isn’t magnetic. The material bonds tungsten and carbon atoms together to create a ceramic-like substance. The magnetic properties come from binding agents used during manufacturing. Most tungsten carbide products we see today are actually composite materials made by sintering tungsten carbide grains with metallic binders.
Effect of Cobalt and Nickel in Tungsten Alloys
A tungsten alloy’s magnetic behavior depends heavily on what binds it together. Iron shows the strongest magnetic pull, cobalt comes second, and nickel has the weakest magnetic response among common binders. Cobalt-tungsten alloys stay ferromagnetic when they contain up to 30% tungsten by weight. The alloys become less plastic and develop higher electrical resistance as their tungsten content rises.
Is Tungsten Steel Magnetic?
Quenched tungsten steel shows remarkable magnetic properties when it contains about 5.5% to 7.0% tungsten with 0.5% to 0.7% carbon. People once used these alloys to make hard permanent magnets because of their high remanence and coercivity. The magnetic features appear because tungsten keeps the martensite phase stable, which shows stronger ferromagnetism than pure iron by resisting magnetic domain wall motion.
Magnetism in Tungsten Rings and Jewelry
Manufacturers make most tungsten rings from tungsten carbide mixed with different amounts of binder metals. The ring’s magnetic properties change based on what goes into it. Rings with cobalt binders will stick to magnets, while those with nickel binders barely react. This becomes crucial for medical reasons – if you need MRI scans, you should choose tungsten jewelry that has minimal magnetic pull. Grades with less binder material react least with magnets, making them a good choice if magnetic properties worry you.
Industrial Applications Where Magnetism Matters
Tungsten’s unique magnetic behavior shows its real value in industries where magnetic properties directly affect performance and functionality.
Tungsten in Aerospace and Defense: Nonmagnetic Advantage
Aerospace and defense sectors value tungsten mainly because it’s non-magnetic and highly dense. Sensitive guidance systems and communication devices work better with tungsten components that prevent magnetic interference. The military uses tungsten alloy bullets instead of depleted uranium. These bullets are better for the environment and don’t have uranium’s radioactive properties. Tungsten also handles extreme temperatures well, which makes it perfect for rocket nozzles and submarine-launched ballistic missiles.
Use in Electronics and CNC Machining
The electronics industry needs tungsten’s carefully controlled magnetic properties. Its high melting point and ability to emit electrons make it great for making bulb filaments and cathode heaters in electronic instruments. CNC machining requires a good understanding of tungsten’s magnetic behavior. Magnetized parts can throw off precise manufacturing processes. Since tungsten is hard and doesn’t conduct heat well, manufacturers often turn to electrical discharge machining (EDM).
Magnetic Considerations in Medical and Tooling Applications
Medical equipment makes good use of tungsten’s controlled magnetic properties, especially in radiation shielding and X-ray technology. MRI-compatible equipment needs non-magnetic tungsten alloys that use nickel instead of cobalt or iron binders. Tungsten-nickel-copper alloys work great in oncology tools and electrical sensor shields because they’re non-magnetic. Tool manufacturers can choose between magnetic and non-magnetic properties. Cobalt binders create magnetic properties, while nickel produces non-magnetic tools.
Conclusion
Tungsten shows us a remarkable example of how atomic structure shapes material properties. The metal is technically paramagnetic with a magnetic susceptibility of +6.8 × 10⁻⁶ emu/g, yet it acts like a non-magnetic material in daily use. This behavior comes from its body-centered cubic crystal structure and electron configuration that stop magnetic domains from forming – domains that would create stronger magnetic responses.
Pure tungsten proves valuable when you need materials with minimal magnetic interference. The story changes with tungsten alloys and carbides. Adding binding metals like cobalt or nickel will substantially change their magnetic properties. These combinations can create materials ranging from strongly magnetic to completely non-magnetic.
Tungsten-based materials’ varied magnetic behaviors make them useful in many industries. The aerospace and defense sectors need tungsten’s components because they won’t affect sensitive guidance systems. Electronics makers depend on tungsten’s controlled magnetic properties for precision work. Medical equipment designers pick specific tungsten alloys that match their magnetic needs, especially when creating MRI-compatible instruments.
The link between tungsten’s composition and magnetic behavior shows why precise engineering matters in metallurgy. Manufacturers can create custom tungsten products that fit specific industrial needs. They might develop non-magnetic components for aerospace or carefully controlled magnetic properties for specialized tools.
Tungsten’s magnetic traits, though subtle, are vital to its industrial flexibility. The metal’s paramagnetic nature pairs with its exceptional physical properties. That combination makes it essential in modern engineering – from aerospace parts and cutting tools to radiation shields and electronic components.
Key Takeaways
Understanding tungsten’s magnetic properties is crucial for engineers and manufacturers selecting materials for precision applications where magnetic interference must be controlled.
• Pure tungsten is technically paramagnetic but behaves as non-magnetic in practice, with extremely weak magnetic susceptibility of +6.8 × 10⁻⁶ emu/g
• Tungsten alloys and carbides exhibit varying magnetic properties depending on binder metals—cobalt creates magnetic behavior while nickel produces non-magnetic materials
• Tungsten’s body-centered cubic crystal structure prevents magnetic domain formation, making it ideal for aerospace and electronics applications requiring zero magnetic interference
• Industrial applications leverage tungsten’s controlled magnetism for MRI-compatible medical devices, precision CNC machining, and sensitive guidance systems in defense equipment
The key insight is that while pure tungsten is essentially non-magnetic, its alloys can be engineered with specific magnetic properties by carefully selecting binder compositions, making tungsten-based materials remarkably versatile across industries requiring precise magnetic control.
FAQs
Q1. Is pure tungsten magnetic? Pure tungsten is technically paramagnetic, meaning it has a very weak positive magnetic susceptibility. However, in practical applications, it behaves essentially as a non-magnetic material due to its extremely weak magnetic response.
Q2. How does tungsten’s magnetic behavior compare to other metals? Unlike ferromagnetic metals like iron or nickel, tungsten’s magnetic susceptibility is several orders of magnitude weaker. This makes tungsten ideal for applications requiring minimal magnetic interference, such as in aerospace and sensitive electronics.
Q3. Can tungsten alloys be magnetic? Yes, tungsten alloys can exhibit magnetic properties depending on their composition. Alloys containing ferromagnetic binders like cobalt can show noticeable magnetic attraction, while those with nickel binders tend to have minimal magnetic response.
Q4. Are tungsten carbide tools magnetic? The magnetism of tungsten carbide tools depends on the binder used in their manufacture. Tools made with cobalt binders will be magnetic, while those made with nickel binders will be non-magnetic. This allows for the production of tools with specific magnetic properties as needed.
Q5. Why is tungsten used in applications requiring non-magnetic materials? Tungsten is used in non-magnetic applications due to its combination of high density, strength, and extremely weak magnetic response. This makes it valuable in industries like aerospace, defense, and medical imaging, where magnetic interference could compromise the performance of sensitive equipment.
