
Did you know that metals can create their shield against corrosion? This remarkable process, called passivation, creates a protective oxide layer on metal surfaces that boosts their resistance to deterioration. Metal surfaces like titanium develop a protective layer up to 25 nm thick over the last several years in air. This natural shield continues to protect without needing any extra treatment.
The passivation process uses nitric acid or citric acid to remove free iron from metal surfaces, especially when you have stainless steel. This creates a stronger protective oxide layer. The process works as a vital part of aerospace and medical device manufacturing because it extends metal components. Stainless steel’s chromium content forms a thin film of chromium oxide when exposed to oxygen. The chrome-to-iron ratio must be 1.5:1 to work properly – this is a big deal as it means that optimal protection against corrosion.
People often mix up passivation with pickling, but they serve different purposes. Pickling aggressively removes oxide scale, while passivation builds up the protective layer without affecting the metal’s appearance. Metal components need passivation after machining operations to restore their protective oxide layer that manufacturing might have damaged.
What is Passivation and Why It Matters in Metal Protection
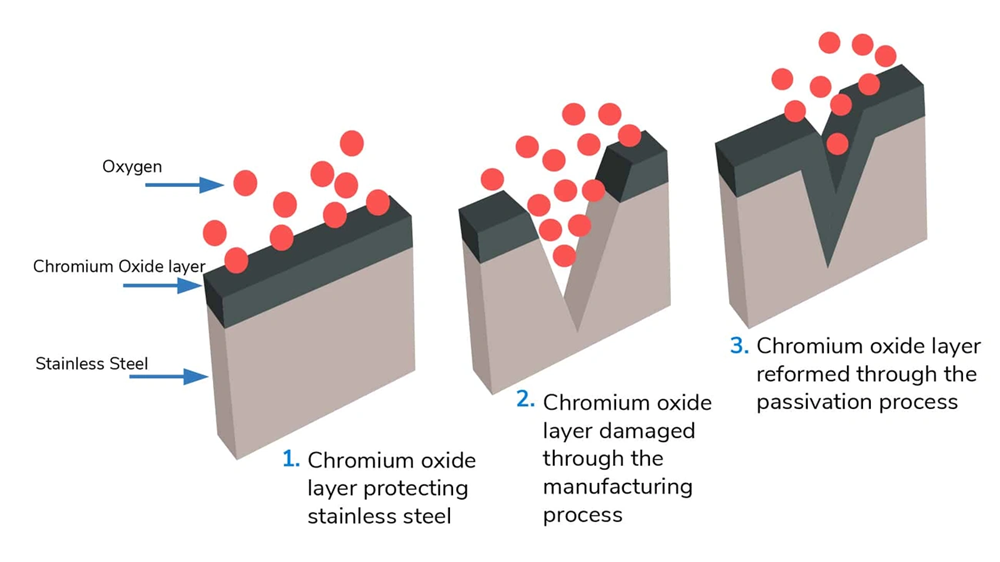
Passivation is a key process in material science that changes how metals interact with their surroundings. This treatment creates exceptional corrosion-resistant properties and extends metal components’ usable life in many industries.
Definition of passivation in chemistry and engineering
Physical chemistry and engineering define passivation as a process that makes materials “passive” and less likely to corrode or react with environmental elements. The process makes certain metals and alloys less chemically reactive, so they act like noble metals such as platinum and gold.
You can create a protective outer layer through three methods: applying a microcoating, starting a chemical reaction with the base material, or letting it oxidize naturally in air. This protective film stops the base material from dissolving and cuts down both chemical and electrical reactivity at the surface.
The barrier keeps oxygen and other corrosive substances away from the metal surface, which means less maintenance for metal parts.
What does passivation do to metal surfaces?
Metal surfaces transform when passivation creates a thin, inert oxide layer that protects against corrosion and oxidation. Stainless steel’s invisible protective film is incredibly thin—about 0.0000001-inch thick, which is 100,000 times thinner than a human hair. This microscopic layer still manages to block corrosive attacks that typically start at exposed surfaces.
The process also removes surface contaminants that can trigger corrosion, including:
- Free iron particles from cutting tools
- Shop dirt containing iron compounds
- Exposed sulfides in free-machining stainless alloys
- Other manufacturing residues that hurt corrosion resistance
The advantages go beyond just protecting against corrosion. Surfaces become cleaner and easier to maintain. Companies also find it easier to comply with regulations in critical sectors like pharmaceuticals, food processing, and medical device manufacturing. Medical applications benefit from passivated surfaces that create stable barriers and prevent metal ions from leaching into the body, which reduces allergic reactions.
Passivated metals develop an amazing ability to heal themselves. Minor scratches trigger immediate oxygen exposure that rebuilds the protective layer. This means ongoing protection without extra treatment.
Difference between natural and chemical passivation
Natural passivation happens on its own when certain metals meet oxygen in the air. This self-passivation needs no human help. Metals like stainless steel, aluminum, chromium, zinc, titanium, and silicon naturally create stable protective oxide layers in air. Stainless steel’s chromium reacts with oxygen to form a passive chromium oxide layer that offers built-in protection.
Chemical passivation takes a different approach. It uses acid solutions—usually nitric or citric acid—to boost the natural process. This treatment cleans surface contaminants better than natural processes and helps form a more even and effective protective layer.
The ASM Specialty Handbook on Stainless Steels points out some debate: “It is not necessary to chemically treat a stainless steel to get the passive film”. Chemical passivation still offers better certainty of maximum corrosion resistance, especially after manufacturing processes that might damage the natural protective layer.
Chemical passivation gives you a top-quality passive film and confidence that finished parts will resist corrosion as much as possible. This makes it a smart choice when corrosion failure could have serious consequences.
How the Passivation Process Works at the Molecular Level
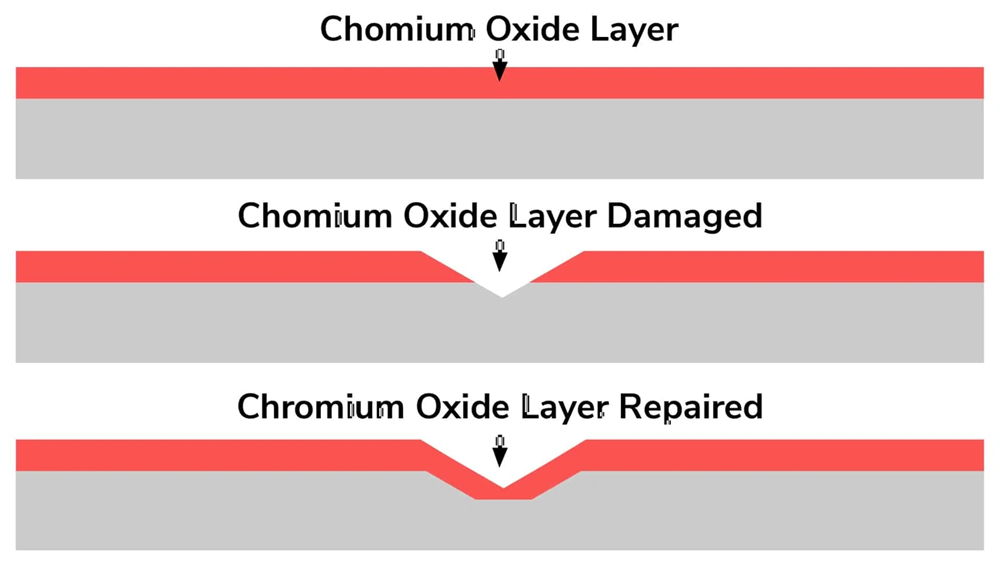
The passivation process involves complex chemical reactions at microscopic levels that change vulnerable metal surfaces into corrosion-resistant barriers. These molecular mechanisms help us understand why some metals resist degradation even in harsh conditions.
Formation of chromium oxide layer in stainless steel
Stainless steel’s resistance to corrosion comes from its chromium content. Chromium reacts with oxygen to create chromium oxide (Cr₂O₃), which forms a protective film on the metal surface. The alloy needs 10.5-12% chromium content to create an effective passive layer.
First principles density functional theory (DFT) calculations show this process goes beyond the surface level. The oxide layer has a specific molecular structure where chromium concentrates at the metal-oxide interface. Research indicates that oxidized chromium exists in the Cr₂O₃ phase, while oxidized iron appears in both FeO and Fe₂O₃ forms.
The passive film has two distinct oxide layers. The outer layer contains chromium oxides, and the inner layer has iron oxides and/or hydroxides. This structure explains stainless steel’s excellent corrosion resistance.
Role of oxygen and acid in oxide layer development
Oxygen is vital to create and maintain the passive layer. It combines with chromium to form chromium oxide on the surface, creating a barrier that stops oxygen and moisture from reaching the iron below. This protective layer, though just a few molecules thick, provides substantial protection.
Chemical passivation speeds up this natural process through acid treatment. The acids serve two purposes:
- They remove free iron and contaminants from the surface
- They promote uniform oxide layer formation
Nitric acid cleans and oxidizes—it dissolves iron compounds while converting chromium to its oxide form. Citric acid removes iron but depends on natural air oxidation to develop the chromium oxide layer.
Passivation layer thickness and stability over time
Different metals develop passive layers of varying thickness. Titanium’s original passivation layer measures about 1 nm, growing to 25 nm after several years in air. Stainless steel’s passive layer measures a few nanometers—about 0.0000001-inch thick, which is 100,000 times thinner than human hair.
Advanced techniques like Auger Electron Spectroscopy (AES) and X-ray Photoelectron Spectroscopy (XPS) show that an effective passive layer needs a minimum chromium-to-iron ratio (Cr/Fe) of 1.3. The oxide layer must be at least 15 Angstroms thick.
This microscopic shield’s stability depends on several factors:
- Environmental conditions
- Temperature fluctuations
- Solution pH
- Exposure duration
- Surface treatment quality
- Ion concentration in the surrounding electrolytes
Properly passivated surfaces can heal themselves. Minor scratches trigger the protective layer to reform when exposed to oxygen, which maintains continuous protection without additional treatment.
Passivation in Stainless Steel: Process, Benefits, and Challenges
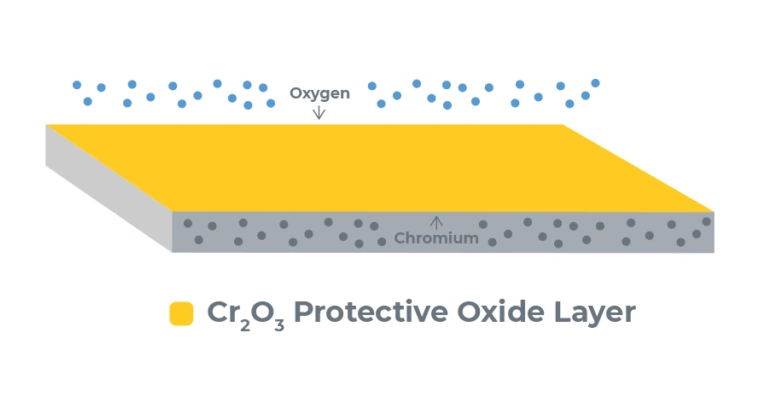
Stainless steel’s amazing corrosion resistance depends on passivation – a most important post-fabrication treatment. This process makes the alloy’s protective properties work at their best. The difference between good performance and early failure in tough applications comes down to this treatment.
What is passivation in stainless steel?
Passivation in stainless steel is a chemical process that removes free iron and surface contaminants. It helps create a protective oxide layer. A clean, freshly machined stainless steel part naturally gets an oxide film from oxygen in the air. This protective film covers all surfaces completely under perfect conditions. Notwithstanding that, we need chemical treatment to improve this natural process for the best corrosion resistance.
The process creates a chromium-rich oxide layer that blocks further oxidation and corrosion of the metal underneath. Passivation isn’t like paint or scale removal – it makes the stainless alloy’s natural corrosion resistance work better.
Removal of free iron and surface contaminants
Tiny amounts of free iron can transfer from cutting tools to stainless steel during machining. The metal might look shiny after machining, but these invisible particles can cause surface rust once exposed to air. There’s another reason – small iron-containing dirt particles from the shop stick to the surface and reduce the protective film’s effectiveness.
Passivation removes exposed sulfides from free-machining stainless alloys’ surfaces, which could start corrosion. You need a thorough cleaning before passivation. All grease, coolant, and shop debris must be removed to get the best corrosion resistance.
Common acids used: nitric vs citric
Two main acids are used to passivate stainless steel:
Nitric acid:
- The original method since the 1960s
- Typical concentration: 20-50% by volume
- Processing time: 20-30 minutes at 120-160°F
- Works as both a cleaner and an oxidizer
Citric acid:
- Coors Brewing Company developed it for beer kegs
- Concentration: 4-10% by weight
- Processing time: 4-60 minutes at 70-140°F
- Safe for the environment and biodegradable
Rouging and flash attack: common problems
Rouging is the biggest problem in stainless steel passivation. Red or black stains appear on surfaces that touch corrosive fluids. Rouge can damage structure, cause system failure, and contaminate products if left alone. This happens when the passive layer gets damaged and lets the metal underneath corrode.
Flash attack happens when chlorides contaminate the passivating solution. Parts don’t come out shiny and bright – they look gray or black instead. Lab tests show that citric acid methods are more likely to cause a flash attack than nitric acid. High bath temperature, too much time in the bath, and contaminated solutions all play a part.
Step-by-Step Breakdown of the Passivation Process
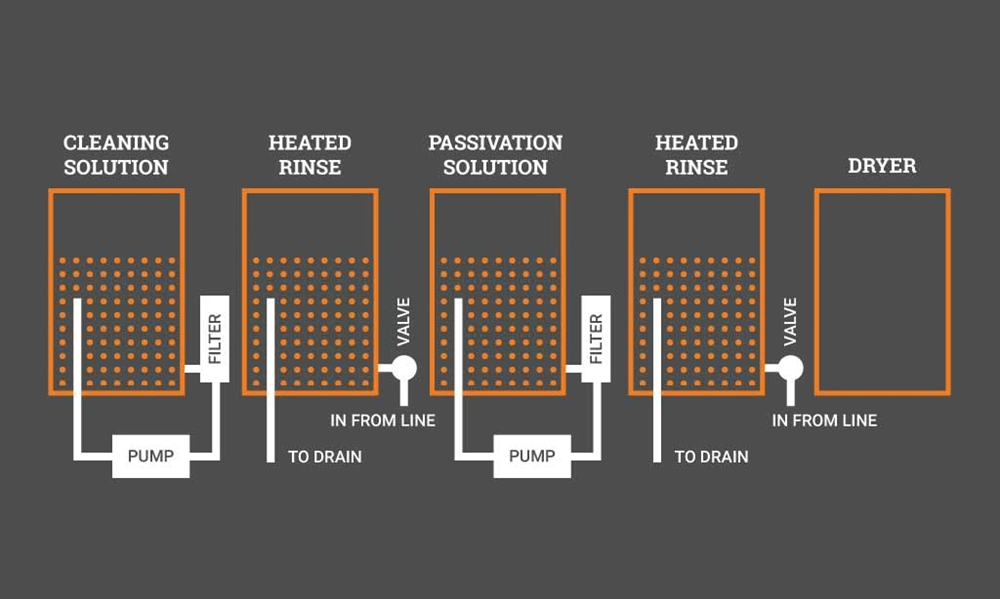
Metal component passivation follows a precise sequence that delivers the best results. Each step creates a corrosion-resistant surface and extends the component’s life. The process needs careful arrangement to work properly.
Alkaline cleaning and degreasing
The first step starts with a complete cleaning to remove contaminants, oils, and foreign materials from the metal surface. This crucial step uses alkaline cleaners like sodium hydroxide, Micro-90, or Simple Green. Ultrasonic cleaning with alkaline solutions works best to remove organic oils and greases. The preliminary degreasing phase must not be skipped. Grease reacts with acid and forms gas bubbles that disrupt passivation when this step is missed.
Acid immersion and oxide layer formation
Clean components go into nitric or citric acid baths. This step dissolves free iron and embedded impurities while speeding up passive oxide film formation. Components stay in the bath for 20-30 minutes at temperatures between 120-150°F. The acid solution must stay free from contaminants. Contamination leads to flash attack that damages the parts’ appearance. Different steel grades should not mix in the same batch.
Rinsing and drying procedures
Components need thorough rinsing with deionized (DI) or reverse osmosis (RO) water to remove acid residue. High-precision industries use cascade or two-step rinsing. The drying process uses lint-free towels, hot air, or nitrogen purging to prevent water spots. Careful handling prevents contamination of the newly passivated surface.
Testing methods: salt spray, copper sulfate, ferroxyl
These testing methods confirm successful passivation:
Salt spray testing puts components in a closed chamber with saltwater fog for 2 to 24 hours. This test shows how well the passive layer holds up in harsh conditions.
The copper sulfate test finds free iron on passivated surfaces in six minutes. Brown or pinkish copper deposits show up if free iron exists. This method works best on austenitic stainless steels that have at least 16% chromium.
The ferroxyl test applies a mix of potassium ferricyanide and nitric acid to the surface. Free iron makes a blue color appear in 30-60 seconds. This test works especially well when checking welded joints.
Industry Standards and Material-Specific Guidelines
Quality standards serve as the backbone of the passivation industry. These standards enforce strict protocols that maintain consistent quality in metal treatments. Companies of all sizes rely on these guidelines for their metal treatment processes.
ASTM A967 and AMS 2700 specifications
The QQ-P-35 military standard from the 1960s led to both ASTM A967 and AMS 2700 specifications. These standards are basically the same but serve different markets. The aerospace sector typically uses AMS 2700, while other industries prefer ASTM A967. Each standard defines the right chemical treatments, concentrations, temperatures, immersion times, and testing methods that work for passivation.
ASTM A967 outlines five nitric acid and five citric acid passivation methods. One example is Nitric 1, which needs “20-25 v% Nitric Acid, 2.5 w% Sodium Dichromate, 120-130°F, 20 Mins minimum”. AMS 2700 focuses on removing free iron from corrosion-resistant steel surfaces.
These standards recommend several testing methods to check if passivation worked properly. Tests include water immersion, high humidity exposure, salt spray, and copper sulfate applications.
Passivation of titanium, aluminum, and silicon
Titanium passivation usually follows ASTM B600 or ASTM F86 guidelines. ASTM F86 only allows nitric acid treatment. ASTM A380 describes nitric acid passivation of titanium and includes descaling options with hydrofluoric acid combinations. Most companies avoid this due to safety concerns.
Aluminum passivation creates a chemically modified oxide “shield” layer. Traditional hexavalent chrome treatments are now replaced by titanium-zirconium systems that are better for the environment. These newer, thinner passivation layers work just as well as chromate conversion processes.
Silicon passivation, common in semiconductor manufacturing, creates stable surfaces that stop electrical leakage.
Citric acid vs nitric acid: safety and environmental impact
Citric acid stands out as the more environmentally friendly choice. Unlike nitric acid, it breaks down naturally, contains no toxins, and produces no harmful fumes. Beer keg manufacturers first developed citric acid passivation. The FDA lists it as Generally Recognized as Safe (GRAS), making it perfect for food and drink applications.
Nitric acid poses serious health risks. Its toxic fumes need proper ventilation. The Environmental Protection Agency classifies it as hazardous waste, requiring special disposal methods. They set a threshold planning quantity at just 500 pounds.
Studies show that 4% citric acid solutions are better for the environment than nitric acid in every way measured. Some applications, especially with titanium, still need nitric acid to get the best results.
Conclusion
The Future and Significance of Metal Passivation
Metal passivation stands out as a breakthrough in science that changes how metals react with corrosive environments. This microscopic shield isn’t visible to our eyes but offers exceptional protection from decay. The process creates self-defending metals through precise chemical reactions that clean contaminants and boost natural protective qualities.
The protective oxide layers from passivation are just nanometers thick—about 100,000 times thinner than a human hair. These ultra-thin layers shield metals effectively from harsh environmental factors. The self-healing nature of well-passivated surfaces provides ongoing protection without extra treatment if small damage happens.
Manufacturers face a crucial choice between nitric and citric acid passivation methods. Nitric acid brings proven reliability from decades of industrial use, while citric acid offers clear environmental benefits. Companies must weigh performance needs against safety factors to pick the right passivation approach.
Product longevity improves dramatically with passivation in many sectors. Medical devices become more biocompatible, aerospace parts stay strong under extreme conditions, and food processing equipment meets strict hygiene rules. Engineers and materials scientists need to understand these basics to get the best performance from metal components.
ASTM A967 and AMS 2700 specifications standardize passivation procedures to ensure quality across industries. These guidelines, plus specific protocols for metals like titanium and aluminum, create clear paths to reliable results. These technical standards lead directly to safer products and longer-lasting critical components.
Metal passivation will keep evolving as environmental rules get stricter and performance demands grow. The core principle stays the same—using natural chemical reactions to create surfaces that resist corrosion and extend component life substantially. This invisible shield proves to be one of material science’s most elegant answers to the persistent problem of metal corrosion.
FAQs
Q1. What is the main purpose of passivation? Passivation is primarily used to remove embedded iron from metal surfaces and enhance corrosion resistance. It creates a protective oxide layer that shields the metal from environmental factors, significantly extending its lifespan and performance.
Q2. How does passivation affect stainless steel? Passivation forms a thin, chromium-rich oxide layer on stainless steel surfaces. This microscopic barrier prevents corrosion, improves chemical resistance, and enhances the metal’s overall durability. Without passivation, stainless steel would be more vulnerable to contamination and degradation.
Q3. What are the key differences between natural and chemical passivation? Natural passivation occurs spontaneously when certain metals are exposed to air, forming a protective oxide layer. Chemical passivation, on the other hand, is an intentional treatment using acid solutions to remove contaminants and create a more uniform and effective protective layer.
Q4. How thick is the passivation layer on metals? The passivation layer is extremely thin, typically measuring just a few nanometers. For example, on stainless steel, it’s approximately 0.0000001-inch thick, which is about 100,000 times thinner than a human hair. Despite its minimal thickness, this layer provides substantial protection against corrosion.
Q5. What are the environmental considerations when choosing between citric and nitric acid for passivation? Citric acid is considered more environmentally friendly as it’s biodegradable, non-toxic, and doesn’t produce harmful fumes. Nitric acid, while effective, presents greater health risks and requires special handling and disposal procedures. The choice between the two often depends on the specific application and environmental regulations.
