
Silver conducts electricity better than gold. This property makes silver plating crucial in modern manufacturing. Scientists in early 19th century Italy developed this electrochemical technique that deposits a thin silver layer on base metals such as copper, steel, titanium, and aluminum. Silver coating’s benefits go well beyond its beautiful appearance. The coating provides excellent protection against corrosion and wear while lasting longer in many industries.
Silver plating has become irreplaceable in today’s manufacturing world. Medical devices like catheters and lasers need biocompatible surfaces that naturally fight bacteria – electroplated silver delivers exactly that. Electronics manufacturers often plate copper components with silver. This method improves conductivity and costs less than using gold or palladium. These practical benefits explain why manufacturers choose silver plating to improve base metal properties instead of using expensive solid silver. Coating thickness can be customized from one micron to 40 microns based on specific industrial needs.
Electroplating Science Behind Silver Coating
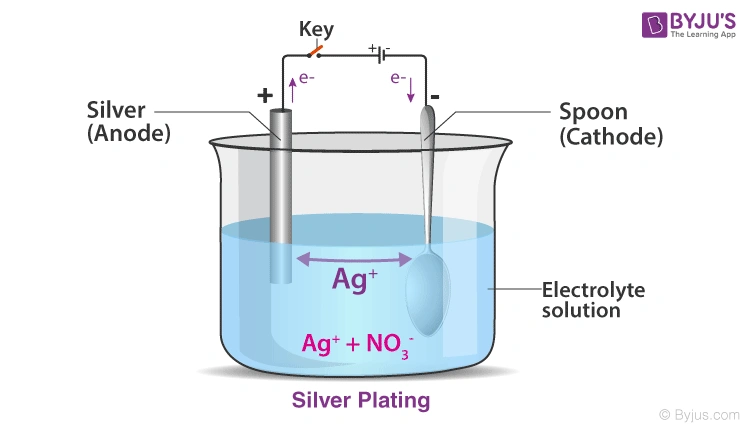
The science of silver coating through electroplating works on basic electrochemical principles. Electric current helps ions move between electrodes through an electrolyte solution, which deposits metal on the substrate surface.
Electrolyte Bath and Anode-Cathode Setup
Silver electroplating uses alkaline cyanide solutions that contain silver cyanide salt, free alkali cyanide, and alkali carbonate. We used potassium-based electrolytes instead of sodium-based ones in most industrial applications because they conduct better and reduce yellowing in the final coating. The solution typically contains silver metal concentration between 10-40 g/l. The object getting plated acts as the cathode and connects to the negative terminal of a power supply. Pure silver or an inert conductive material serves as the anode.
Ion Transfer and Deposition Mechanism
The process revolutionizes as oxidation happens at the anode, which changes solid silver into dissolved silver ions (Ag+). These positive ions travel through the electrolyte solution toward the negative cathode. The ions gain electrons at the cathode’s surface and turn back into metallic silver, creating a thin layer on the substrate. This effectively moves silver from the anode to the cathode.
Silver anodes gradually dissolve to replenish metal ions in the solution. So, silver transfers from the anode to the cathode. On top of that, the bath’s free cyanide performs vital functions: it keeps silver soluble, provides electrical conductivity, and helps dissolve the anode.
Role of Current Density in Layer Uniformity
Current density – the amount of electrical current flowing through an area – affects the coating’s thickness and quality directly . Higher current density creates thicker coatings as more metal ions deposit. But too much current density can cause uneven deposition, porosity, and other defects.
The plating bath’s “throwing power” measures how uniformly current distributes, which determines how even the electroplated silver thickness becomes. This depends on the electrolyte’s composition, temperature, and operating current density. Most silver plating operations work best with cathode current density between 1-5 A/dm².
Types of Silver Plating Techniques in Industry

Manufacturing today uses three main silver plating techniques. Companies choose these methods based on component size, geometry, and production volume needs.
Barrel Plating for Small Components
Silver plating in industry often employs barrel plating to handle many smaller parts at once. This method puts components inside a barrel-shaped cage made from non-conductive materials and submerges it in an electrolytic bath with silver ions. The barrel’s slow rotation helps individual pieces make bipolar contact with each other, which results in better plating efficiency and even coverage on all surfaces.
Barrel plating has clear advantages. It processes large volumes of parts simultaneously and saves time and money. The whole process—cleaning, rinsing, pickling, and sealing—happens in a single vessel, which reduces handling needs. In spite of that, barrel plating doesn’t work well when parts need decorative finishes or must meet specific engineering requirements.
Rack Plating for Complex Geometries
Parts with complex shapes or delicate features work better with rack plating techniques. This approach uses specially designed racks with hooks, screws, or bands that hold items securely while providing electrical conductivity. The rack then goes into the silver plating solution.
Rack processes improve conductivity, corrosion resistance, and solderability compared to barrel plating. They also allow selective plating buildup and more even silver distribution. The process costs more and needs more labor since workers must position and remove each part individually, but it creates better-looking finishes and works well with fragile components. The main drawback shows up as “rack marks”—small unplated spots where parts touch the rack fixtures.
Reel-to-Reel Plating for High-Volume Production
Reel-to-reel plating streamlines processes for large production volumes with precision. This automated system moves material through various electroplating processes using capstan mechanisms and take-up systems that re-spool the finished product. Masking methods help achieve selective deposition by covering areas that don’t need plating.
These systems excel at making electronic components, especially connectors used in telecommunications. Their high capacity, reliability, and precision make them perfect for electronics, medical device, and automotive industries. Advanced features include modular construction, interchangeable cells, and computerized inspection capabilities. Setup takes longer than other methods, but the benefits outweigh this drawback.
Material Compatibility and Pre-treatment Requirements
Material selection greatly affects the outcome of silver plating processes. Different substrate metals need specific preparation techniques. Each base material brings its own set of challenges that need solutions before applying electroplated silver.
Silver Plating Copper and Aluminum
Copper and copper alloys generally accept silver plating easily. Pure copper grades (C101 and C110) need only alkaline cleaning and acid pickling to remove surface oxides. Tellurium copper needs special attention because its 0.5% tellurium content reacts with cyanide solutions. This reaction forms insoluble compounds that stop proper adhesion.
Aluminum creates very different challenges because it oxidizes quickly in air. A specialized zincate process solves this by stripping aluminum oxide while laying down a thin zinc layer. Best results come from a double zincate application that creates a uniform “metallic shrink wrap” to prevent reoxidation. An electroless nickel strike then removes the zinc layer. This forms a very strong bond with the aluminum substrate—a method that works better than other copper strike approaches.
Nickel Undercoating for Steel and Brass
Steel, zinc, and zinc-based alloys need a nickel-over-copper undercoat before silver plating. Copper and copper alloys also need nickel undercoating, but for different reasons. Nickel stops a weak silver-copper eutectic from forming at temperatures above 149°C (300°F). High-temperature applications suffer from weak adhesion without this barrier layer.
A modified Woods nickel process works well for stainless steel and high-temperature alloys containing chromium, nickel, and cobalt. This process removes tough passive oxide films while laying down an active nickel layer. Brass with higher zinc content (like C360 with 37% zinc) needs a duplex acid pickle. This first removes standard copper/zinc oxides, then tackles surface lead inclusions.
Surface Cleaning and Activation Steps
Surface preparation has three vital phases:
- Pre-cleaning: Removes bulk contaminants through soaking in alkaline solutions adjusted to the base material
- Secondary cleaning: Eliminates microscopic traces through polishing via abrasive blasting, ultrasonic washing, or spray techniques
- Surface activation: Removes oxide layers, particularly important for stainless steel and nickel-containing alloys
The main goal aims to achieve a “water break free” surface—where water forms a continuous sheet instead of beading up. This indicates complete removal of oils and contaminants. Experts say that “the quality of an electrodeposit is only as good as the condition of the underlying surface”. This shows why proper preparation directly determines plating success.
Performance Characteristics of Electroplated Silver
Silver plating delivers outstanding benefits that make it a top choice for industrial use. The unique properties of silver coating bring great value to many applications.
Electrical Conductivity vs. Gold
Silver stands out as the most conductive element we know, with a conductivity rating of 6.2×10^7 S/m. This rating beats copper (5.9×10^7 S/m) and is much higher than gold’s conductivity (4.5×10^7 S/m) by about 38%. Gold remains the preferred choice for some electronics because it resists corrosion better. Silver’s great electrical properties make it perfect for printed circuit boards, connectors, and semiconductors. The metal keeps its excellent conductivity even after tarnishing.
Corrosion Resistance in Harsh Environments
Silver shows good corrosion resistance but can be weak in certain conditions. The metal handles hot, moist air and ammonia exposure well. However, it reacts quickly with sulfur compounds and creates silver sulfide (Ag₂S) – the black coating we call tarnish. This reaction affects conductivity a lot since silver sulfide’s resistivity is about 100,000 times higher than pure silver. The coating thickness plays a key role in fighting corrosion. Thicker, pore-free coatings (around 20 μm) stop the copper underneath from affecting how corrosion happens.
Solderability and Thermal Stability
Silver plating shows excellent solderability characteristics that work great for electronics manufacturing. The Sn62 alloy (tin-lead-silver) works best when soldering to silver-plated surfaces. Good solderability needs plating without pores and chemical contamination. The metal performs well at high temperatures and can handle heat up to 1200°F (649°C). Nickel-enhanced silver coatings keep stable electrical resistance at 165°C for long periods in special uses.
Lubricity and Anti-Galling Properties
Silver plating’s special advantage comes from its great lubricity, especially in high heat. This makes silver coating valuable for anti-galling uses like bearings, fasteners, gears, and nuts. Silver works as a lubricant even in liquid form and stops parts from seizing. This anti-seizing feature helps static seals, bushings, and sliding components work better. Electroplated silver’s hardness ranges from 90 to 135 Brinell based on plating conditions. This creates the right balance between wear resistance and smooth performance.
Conclusion
Silver plating is a remarkable metal finishing process that blends scientific principles with practical industrial uses. This electrochemical technique turns ordinary base metals into components with extraordinary properties. We explored in this piece how silver plating works through ion transfer mechanisms in electrolyte baths. Current density plays a vital role in determining the coating’s quality.
Manufacturing today uses three different approaches. Barrel plating works best for small components, rack plating handles complex geometries, and reel-to-reel systems tackle high-volume production. Each method has unique advantages based on component needs and production targets. The bond between silver and various substrate materials needs careful preparation, especially when you have reactive metals like aluminum or specialized alloys.
Silver coatings deliver exceptional performance that explains their wide use in industry. Silver’s electrical conductivity surpasses even gold, which makes it a great choice for electronics. While it can be vulnerable to sulfur compounds, silver shows strong resistance to corrosion in many harsh environments with the right coating thickness. Silver-plated parts also offer excellent solderability, thermal stability up to 649°C, and remarkable smoothness that prevents galling between metal surfaces.
Silver plating’s versatility makes it essential across industries. Medical devices benefit from silver’s biocompatibility, and electronic components leverage its superior conductivity. This time-tested technique balances performance with budget-friendly solutions. Without doubt, silver plating remains indispensable in modern manufacturing and provides customized solutions where conductivity, corrosion resistance, and durability matter most.
FAQs
Q1. How durable is silver plating? The durability of silver plating depends on several factors, including the quality of the plating process, frequency of use, and storage conditions. With proper care, such as storing in a dry, airtight container and avoiding exposure to harsh chemicals, silver plating can last for many years. However, it’s important to note that silver plating will eventually wear off with regular use.
Q2. What is the typical cost of silver replating? The cost of silver replating varies based on the size and type of the item. For example, replating a small set like a creamer and sugar bowl might cost around $40, while a medium-sized tray could range from $22 to $25. Larger items such as teapots may cost between $30 to $40 to replate. Prices can vary depending on the service provider and the complexity of the item.
Q3. Does electroplated silver have any value? While electroplated silver items contain only a thin layer of silver, their value lies more in their esthetic appeal, craftsmanship, and potential historical significance rather than their silver content. Antique or well-preserved pieces, especially those from renowned manufacturers or with unique designs, can be valuable to collectors. However, the silver content alone is typically not significant enough for melting down.
Q4. Can silver plating wear off over time? Yes, silver plating can wear off over time. The thin layer of silver applied during the electroplating process is susceptible to wear and tear from regular use, exposure to moisture, and contact with skin oils. While proper care and maintenance can slow down this process, eventually, the plating may show signs of wear or rubbing off, particularly on frequently used areas of an item.
Q5. What are the key performance characteristics of electroplated silver? Electroplated silver offers several notable performance characteristics. It provides excellent electrical conductivity, even surpassing gold in this aspect. Silver plating also offers good corrosion resistance in many environments, though it is susceptible to tarnishing from sulfur compounds. Additionally, it demonstrates superior solderability, thermal stability at high temperatures, and exceptional lubricity, making it valuable for various industrial and electronic applications.
